|
Saponifiable and Nonsaponifiable Lipids
Fats or lipids can be considered to be biological molecules which are soluble in organic solvents, such
as chloroform/methanol, and are sparingly soluble in aqueous solutions. There are two major classes, saponifiable and nonsaponifiable,
based on their reactivity with strong bases. The nonsaponifiable classes include the fat-soluble
vitamins (A, E) and cholesterol.
Figure: Examples saponifiable and nonsaponifiable lipids
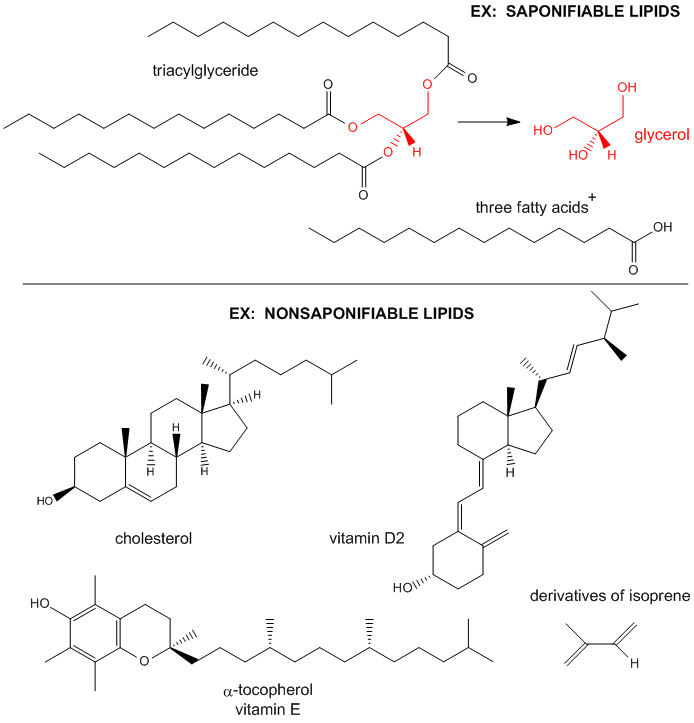
Saponification is the process that produces soaps from the reaction of fats and a strong base.
The saponifiable lipids contain long chain carboxylic acids, or fatty acids, esterified to a “backbone”
molecule, which is either glycerol or spinghosine.
The major saponifiable lipids are triacylglycerides, glycerophospholipids, and the sphingolipids. The
former two use glycerol as the backbone. Triacylglycerides have three fatty acids esterified to the three OHs on glycerol.
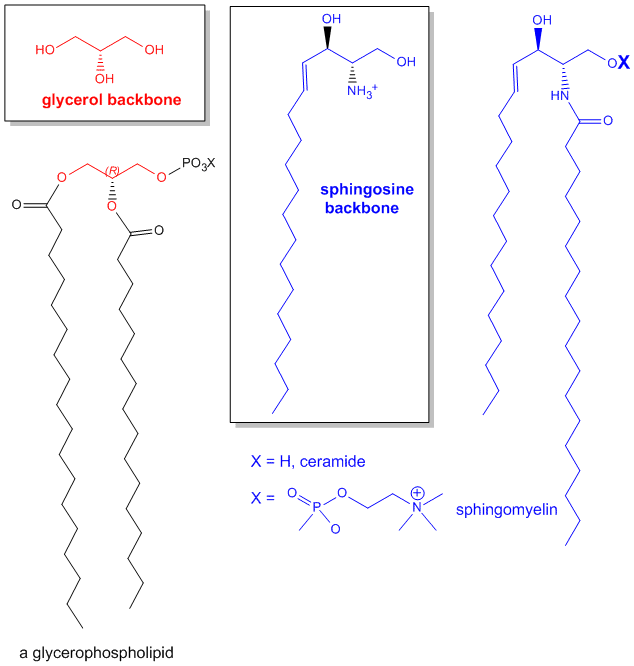
Glycerophospholipids have two fatty acids esterified at carbons 1 and 2, and a phospho-X groups esterifed at C3. Sphingosine,
the backbone for the sphingolipids, has a long chain alkyl group connected at C1, a fatty acid in an amide link at C2, and
a H or phospho-X group esterified at C3. Diagrams showing these structures are given below.
Figure: Classification of common phosopholipids, glycolipids, and triacylglyerides
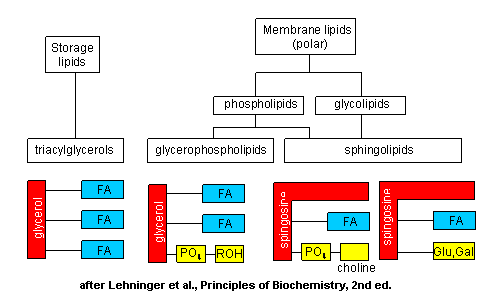
Figure: Structures of common phospholipids
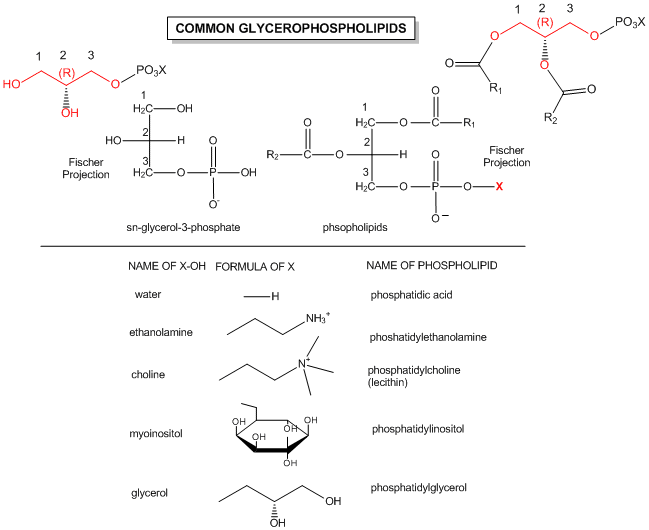
Figure: Comparison of lipids with glycerol and sphingosine
as backbones
Properties of Lipids
The structure of these molecules determines their function. For example, the very insoluble triacylglycerides
are used as the predominant storage form of chemical energy in the body. In contrast to polysaccharides such as glycogen
(a polymer of glucose), the Cs in the acyl-chains of the triacylglyceride are in a highly reduced state. The main source of
energy to drive not only our bodies but also our society is that obtained through oxidizing carbon-based molecules to carbon
dioxide and water, in a reaction which is highly exergonic and exothermic. Sugars are already part way down the free energy
spectrum since each carbon is partially oxidized. 9 kcal/mol can be derived from the complete oxidation of fats, in contrast
to 4.5 kcal/mol from that of proteins or carbohydrates. In addition, glycogen is highly hydrated. For every 1 g of glycogen,
2 grams of water is H-bonded to it. Hence it would take 3 times more weight to store the equivalent amount of energy in carbohydrates
as is stored in triacylglyceride, which are stored in anhydrous lipid "drops" within cells . The rest of this unit on
lipids will focus not on triacylglycerides, whose main function is energy storage, but on fatty acids and phospholipids, and
the structures they form in aqueous solution.
The structure of fatty acids and phospholipids show them to amphiphilic - i.e. they have both hydrophobic
and hydrophilic domains. Fatty acids can be represented in "cartoon-form" as single chain amphiphiles with a circular polar
head group and a single acyl non-polar tail extending from the head. Likewise, phospholipids can be shown as double chain
amphiphiles. Even cholesterol can be represented this way, with its single OH group as the polar head, and the rigid 4 member
rings as the hydrophobic “tail”. Even through there are a very large number of fatty acids which can be esterified
to C1 and C2 of phospholipids and a variety of P-X groups at C3, making the phospholipids and fatty acids extremely
heterogeneous groups of molecules, their role in biological structures can be understood quite simply by modeling them either
as single or double chain amphiphiles. This reduces their apparent complexity dramatically. In addition, they, in contrast
to carbohydrates, amino acids, and nucleotides, do not form covalent polymers. Hence we will start our studies of biological
molecules with lipids (fatty acids and phospholipids) and then apply our understanding of this class of molecules to the more
complex systems of biological polymers. We will see that phospholipids and sphingolipids are essential components of
membrane structure. Cholesterol is also found in membranes and is a precursor of steroid hormones.
Fatty acid structure and conformation
Fatty acids can be saturated (contain no double bonds in the acyl chain), or unsaturated (with
either one -monounsaturated - or multiple - polyunsaturated - double bond(s)) . The following diagram shows the
relative conformations of saturated and unsaturated fatty acids. We will explore this diagram a bit latter.
Table: Names and structures of the most common fatty acids
COMMON BIOLOGICAL SATURATED FATTY ACIDS
|
Symbol |
common name |
systematic name |
structure |
mp(C) |
|
12:0 |
Lauric acid |
dodecanoic acid |
CH3(CH2)10COOH |
44.2 |
|
14:0 |
Myristic acid |
tetradecanoic acid |
CH3(CH2)12COOH |
52 |
|
16:0 |
Palmitic acid |
Hexadecanoic acid |
CH3(CH2)14COOH |
63.1 |
|
18:0 |
Stearic acid |
Octadecanoic acid |
CH3(CH2)16COOH |
69.6 |
|
20:0 |
Arachidic aicd |
Eicosanoic acid |
CH3(CH2)18COOH |
75.4 |
COMMON BIOLOGICAL UNSATURATED FATTY ACIDS
|
Symbol |
common name |
systematic name |
structure |
mp(C) |
|
16:1D9 |
Palmitoleic acid |
Hexadecenoic acid |
CH3(CH2)5CH=CH-(CH2)7COOH |
-0.5 |
|
18:1D9 |
Oleic acid |
9-Octadecenoic acid |
CH3(CH2)7CH=CH-(CH2)7COOH |
13.4 |
|
18:2D9,12 |
Linoleic acid |
9,12 -Octadecadienoic acid |
CH3(CH2)4(CH=CHCH2)2(CH2)6COOH |
-9 |
|
18:3D9,12,15 |
a-Linolenic
acid |
9,12,15 -Octadecatrienoic acid |
CH3CH2(CH=CHCH2)3(CH2)6COOH |
-17 |
|
20:4D5,8,11,14 |
arachidonic acid |
5,8,11,14- Eicosatetraenoic acid |
CH3(CH2)4(CH=CHCH2)4(CH2)2COOH |
-49 |
|
20:5D5,8,11,14,17 |
EPA |
5,8,11,14,17-Eicosapentaenoic- acid |
CH3CH2(CH=CHCH2)5(CH2)2COOH |
-54 |
|
22:6 D4,7,10,13,16,19 |
DHA |
Docosohexaenoic acid |
22:6w3 |
|
% FATTY
ACIDS IN VARIOUS FATS
|
FAT |
<16:0 |
16:1 |
18:0 |
18:1 |
18:2 |
18:3 |
20:0 |
22:1 |
22:2 |
. |
|
Coco-nut |
87 |
. |
3 |
7 |
2 |
. |
. |
. |
. |
. |
|
Canola |
3 |
. |
|
11 |
13 |
10 |
. |
7 |
50 |
2 |
|
Olive Oil |
11 |
. |
4 |
71 |
11 |
1 |
. |
. |
. |
. |
|
Butter-fat |
50 |
4 |
12 |
26 |
4 |
1 |
2 |
. |
. |
. |
Figure: Conformations of fatty acids
Fatty acids can be named in many ways.
- symbolic name: given as x:y (D a,b,c) where x is the number of C’s in the chain, y is the number of double
bonds, and a, b, and c are the positions of the start of the double bonds counting from C1 - the carboxyl C. Saturated fatty
acids contain no C-C double bonds. Monounsaturated fatty acids contain 1 C=C while polyunsaturated fatty acids contain
more than 1 C=C. Double bonds are usual cis.
- systematic name using IUPAC nomenclature. The systematic name gives the number of Cs (e.g. hexadecanoic
acid for 16:0). If the fatty acid is unsaturated, the base name reflects the number of double bonds (e.g. octadecenoic acid
for 18:1 D 9 and
octadecatrienoic acid for 18:3D 9,12,15).
- common name: (e.g. oleic acid, which is found in high concentration in olive oil)
You should know the common name, systematic name, and symbolic representations for these saturated fatty:
- lauric acid, dodecanoic acid, 12:0
- palmitic acid, hexadecanoic acid, 16:0
- stearic acid, octadecanic acid, 18:0.
Learn the following unsaturated fatty acids -
- oleic acid, octadecenoic acid, 18:1 D
9
- linoleic acid, octadecadienoic acid, 18:2 D 9,12
- a-linolenic acid, octadecatrienoic acid, 18:3 D 9,12,15 (n-3)
- arachidonic acid, eicosatetraenoic acid, 20:4 D 5,8,11,14 (n-6)
- eicosapentenoic acid (EPA), 20:5 D
5,8,11,14,17 (n-3) Note: sometimes written as eicosapentaenoic
- docosahexenoic acid (DHA) 22:6 D4,7,10,13,16,19
(n-3) Note: sometimes written as docosahexaenoic
There is an alternative to the symbolic representation of fatty acids, in which the Cs are numbered from
the distal end (the n or w end) of the acyl chain (the opposite end from
the carboxyl group). Hence 18:3 D 9,12,15 could be written as 18:3 (w -3) or 18:3 (n
-3) where the terminal C is numbered one and the first double bond starts at C3. Arachidonic
acid is an (w -6) fatty acid while docosahexaenoic acid is an (w -3) fatty acid.
Note that all naturally occurring double bonds are cis, with a methylene spacer between double bonds -
i.e. the double bonds are not conjugated. For saturated fatty acids, the melting point increases with C chain length, owing
to increased likelihood of van der Waals (London or induced dipole) interactions between the overlapping and packed chains.
Within chains of the same number of Cs, melting point decreases with increasing number of double bonds, owing to the kinking
of the acyl chains, followed by decreased packing and reduced intermolecular forces (IMFs). Fatty acid composition differs
in different organisms:
- animals have 5-7% of fatty acids with 20-22 carbons, while fish have 25-30%
- animals have <1% of their fatty acids with 5-6 double bonds, while plants have 5-6% and fish 15-30%
Many studies support the claim the diets high in fish that contain abundant n-3 fatty acids, in particular EPA and DHA,
reduce inflammation and cardiovascular disease. n-3 fatty acids are abundant in high oil fish (salmon, tuna, sardines),
and lower in cod, flounder, snapper, shark, and tilapia.
The most common polyunsaturated fats (PUFAs) in our diet are the n-3 and n-6 classes. Most abundant in the
n-6 class in plant food is linoleic acid (18:2n-6, or 18:2D9,12), while linolenic acid
(18:3n-3 or 18:3D9,12,15) is the most abundant in the n-3 class. These fatty acids
are essential in that they are biological precursors for other PUFAs. Specifically,
- linoleic acid (18:2n-6, or 18:2D9,12) is a biosynthetic precursor of arachidonic acid
(20:4n-6 or 20:4D5,8,11,14)
- linolenic acid (18:3n-3, or 18:3D9,12,15) is a biosynthetic precursor of eicosapentaenoic
acid (EPA, 20:5n-3 or 20:5D5,8,11,14,17) and to a much smaller extent, docosahexaenoic
acid (DHA, 22:6n-3 or 22:6D4,7,10,13,16,19).
These essential precursor fatty acids are substrates for elongases, desaturases, and beta-oxidation type enzymes in the
endoplasmic reticulum and another organelle, the peroxisome (involved in oxidative metabolism of straight chain and branched
fatty acids, peroxide metabolism, and cholesterol/bile salt synthesis). Animals fed diets high in plant 18:2(n-6) fats
accumulate 20:4(n-6) fatty acids in their tissues while those fed diets high in plant 18:3(n-3) accumulate 22:6(n-3).
Animals fed diets high in fish oils accumulate 20:5 (EPA), 22:5 and 22:6 (DHA) at the expense of 20:4(n-6).
Recent work has suggested that contrary to images of early hominids as hunters and scavengers of meat, human brain development
might have required the consumption of fish which is highly enriched in arachidonic and docosahexaenoic acids. A large
percent of the brain consists of lipids, which are highly enriched in these two fatty acids. These acids are necessary
for the proper development of the human brain and in adults, deficiencies in these might contribute to cognitive disorders
like ADHD, dementia, and dyslexia. These fatty acids are essential in the diet, and probably could not have been derived in
high enough amounts from the eating of brains of other animals. The mechanism for the protective effects of n-3
fatty acids in health will be explored later in the course when we discuss prostaglandins synthesis and signal transduction.
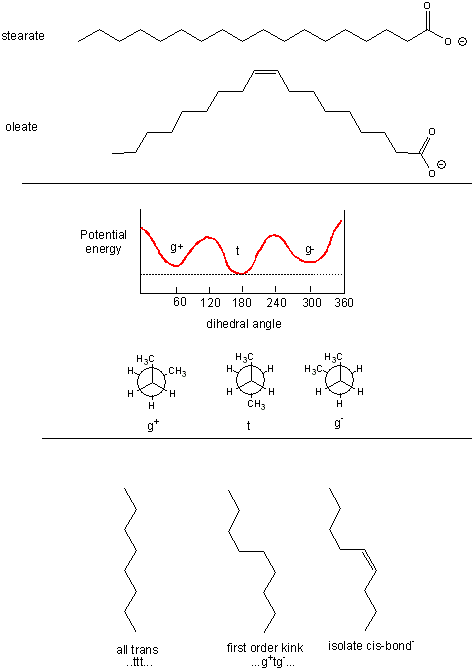
Saturated fatty acids chains can exist in many conformations resulting from free rotation around the C-C
bonds of the acyl chains. A quick review of the conformations of n-butane shows that the energetically most favorable conformation
is one in which the two CH3 groups attached to the 2 methylene C’s (C2 and C3) are trans to each other, which
results in decreased steric strain. Looking at a Neuman projection of n-butane shows the dihedral or torsional angle of this
trans conformation to be 180 degrees. When the dihedral angle is 0 degrees, the two terminal CH3 groups are syn
to each other, which is the conformation of highest energy. When the angle is 60 (gauche+) or 300 (gauche-) degrees, a higher,
local minimum is observed in the energy profile. At a given temperature and moment, a population of molecules of butane
would consist of some in the g+ and g- state, with most in the t state. The same applies to fatty acids. To increase the number
of chains with g+tg- conformations, for example, the temperature of the system can be increased.
NEXT >>
|

