|
We have previously discussed how chemical potential energy in the form of reduced organic molecules can
be transduced into the chemical potential energy of ATP. This ATP can be used to drive reductive biosynthesis and movement
(from individual cells to whole organisms). ATP has two other significant uses in the cell.
Active Transport: Molecules must often move across membranes against a concentration
gradient - from low to high chemical potential - in a process characterized by a positive DG. As protons could be "pumped" across the inner mitochondrial membrane against a concentration gradient,
powered by the DG associated with electron transport (passing electrons from
NADH to dioxygen), other species can cross membranes against a concentration gradient - a process called active transport
- if coupled to ATP hydrolysis or the collapse of another gradient. This active transport is differentiated from facilitated
diffusion we studied earlier, which occurred down a concentration gradient across the membrane. Many
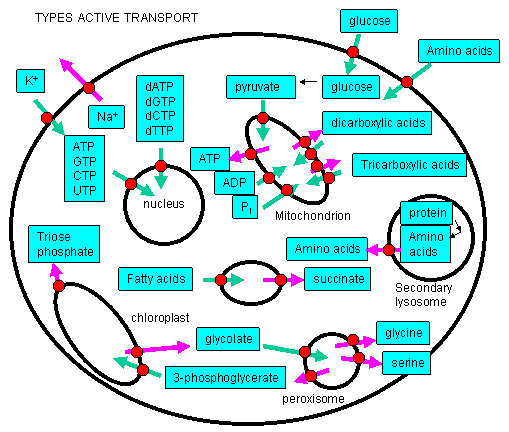
such species must be transported into the cell or into intracellular organelles against a concentration gradient!
Figure: Many such species must be transported into the cell or into intracellular organelles against a concentration gradient
Signal Transduction: All cells must know how to respond to their environment. They
must be able to divide, grow, secrete, synthesize, degrade, differentiate, cease growth, and even die when the appropriate
signal is given. This signal invariably is a molecule which binds to a receptor, typically on the cell surface. (Exceptions
include light transduction in retinal cells when the signal is a photon, and lipophilic hormones which pass through the membrane.)
Binding is followed by shape changes in transmembrane protein receptors which effectively transmits the signal into the cytoplasm.
We will discuss three main types of signal transduction pathways:
- nerve conduction, in which a presynaptic neuron releases a neurotransmitter causing a postsynaptic neuron
to "fire";
- signaling at the cell surface which leads to activation of kinases within the cytoplasm;
- apotosis or programmed cell death
We will discuss signal transduction in the final three sections.
Energy Requirements for Active Transport.
For active transport to occur, a membrane receptor is required which recognizes the ligand to be transported.
Of major interest to us, however, is the energy source used to drive the transport against a concentration gradient. The biological
world has adapted to use almost any source of energy available.
- Energy released by oxidation: We have already encountered the active transport of protons
driven by oxidative processes. In electron transport in respiring mitochondria, NADH is oxidized as it passes electrons to
a series of mobile electron carriers (ubiquione, cytochrome C, and eventually dioxygen) using Complex 1, 3 and 4 in the inner
membrane of the mitochondria. Somehow the energy lost in this thermodynamically favored process was coupled to conformational
changes in the complex which caused protons to be ejected from the matrix into the inner membrane space. One can imagine a
series of conformation-sensitive pKa changes in various side chains in the complexes which lead in concert to the
vectorially discharge of protons.
- ATP hydrolysis: One would expect that this ubiquitous carrier of free energy would by used
to drive active transport. In fact, this is one of the predominant roles of ATP in the biological world. 70% of all ATP turnover
in the brain is used for the creation and maintenance of a Na and K ion gradient across nerve cell membranes using the membrane
protein Na+/K+ ATPase.
- Light: Photosynthetic bacteria have a membrane protein called bacteriorhodopsin which contains
retinal, a conjugated polyene derived from beta-carotene. It is analogous to the visual pigment protein rhodopsin in
retinal cells. Absorption of light by the retinal induces a conformation changes in the retinal and protein, which leads to
vectorial discharge of protons ;
- Collapse of an ion gradient: The favorable collapse of an ion gradient can be used to drive
the transport of a different species against a concentration gradient. We have already observed that collapse of a proton
gradient across the inner mitochondria membrane (through FoF1ATPase)
can drive the thermodynamically unfavored synthesis of ATP. Collapse of a proton gradient provides a proton-motive
force which can drive the active transport of sugars. Likewise a sodium-motive force can
drive active transport of metal ions. Since the energy to make the initial ion gradients usually comes from ATP hydrolysis,
ATP indirectly powers the transport of the other species against a gradient.
Types of Active Transport
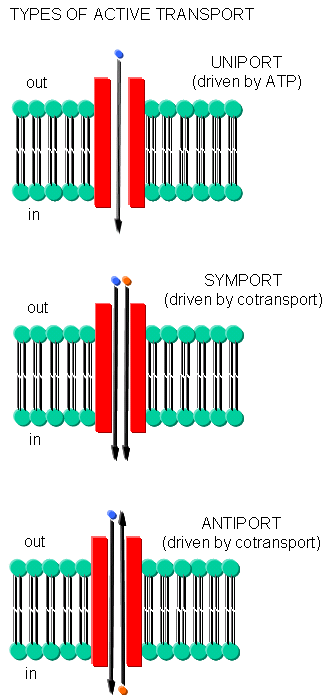
Often times, transport of one species is coupled to transport of another.
If the species are charged, a net change in charge across the membrane may occur. Several terms are used to describe
various types of transport:
- symport - two species are cotransported in the same direction by the same transport protein
- antiport - two species are cotransported in opposite directions by the same transport protein
- electrogenic - a net electrical imbalance is generated across the membrane by symport or
antiport of charged species
- electroneutral - no net electrical imbalance is generated across the membrane by symport
or antiport of charged species
Figure:
Examples of Transport: Metal Ions
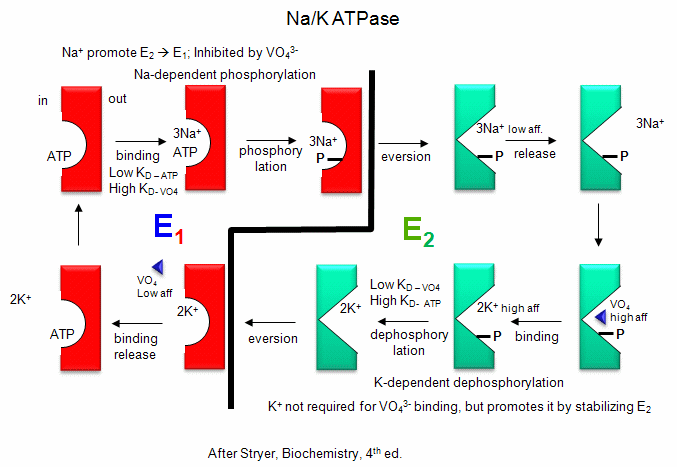
- Na/K - These ions are both transported by the Na/K ATPase. This protein keeps
the K+in and Na+out high compared to their respective concentrations on the other
side of the membrane. The protein exists in two essential conformations, E1 and E2, depending on the phosphorylation state
of the protein. ATP and 3 Na+ bind to the cytoplasmic domain of the enzyme in the E1 conformation. In the presence
of Na ions, the bound ATP is cleaved in a nucleophilic atack by an Asp side chain of the protein. (Hence, the protein is a
Na+-activated ATPase. The phosphorylated enzyme changes conformation to the E2 form in which Na+ ions
are now on the outside of the cell membrane, from which they dissociate. The phosphorylated protein in conformation E2 now
binds 2 K+ ions on the outside, which activates hydrolysis of the Asp-PO3 mixed anhydride link. The
dephosphorylated protein is more stable in the E1 conformation to which it changes as it bring K+ ions into the
cell. This is an example of an electrogenic antiporter. Transport proteins that use this mechanism of transport are
designated as P types, since ATP cleavage is required and PO43- is covalenty transferred to
an Asp residue from the ATP. P-Type transporters are inhibited by vanadate, a transition state analog of
phosphate. Note: Transport mediated by P type membrane proteins can, in the lab, be used to drive ATP synthesis.
Na/K-ATPase.
Figure: Na/K-ATPase
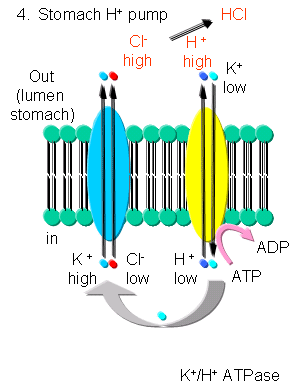
- K - In addition to the above mechanism, K ions can be transported with protons in an electroneutral
antiport mechanism by a K+/H+-ATPase found in stomach cells, which gives rise to a low pH in the lumen
of the stomach.
Figure: K/H ATPase
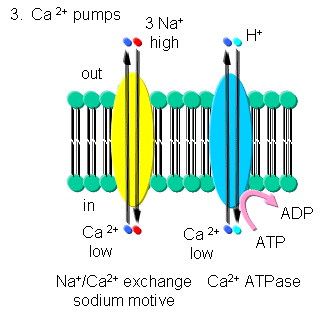
- Ca - Calcium levels are very low in cells. Transient increases are more likely to be detected
in a signal transduction pathways than a transient decrease in high basal or constituitive cytoplasmic levels. Ca2+-ATPase,
homologous to the Na/K-ATPase, removes Ca2+ from the cytoplasmic to either the outside of the cells or into internal
organelles. In addition a Na+-Ca2+ exchange protein (an antiporter) transports calcium ions out of the
cell using a sodium-motive potential.
Transport of calcium ions
Figure: Transport of calcium ions
All of above ATPases are examples of P-type ion transporters. There are also other types. F-type
are similar to the F0F1ATPases and can transport protons against a concentration gradient powered
by ATP breakdown. Notice that this is the opposite role for this enzyme that we discussed in mitochondrial oxidative
phosphorylation. V-type (vacuolar) are found in the membranes of acidic organelles (like lysosomes)
and acidic vesicles within neurons, where neurotransmitters are stored.
Examples of Transport: Sugars
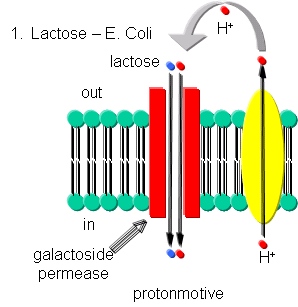
- Lactose - Lactose can be transported into E. Coli against a concentration gradient
using galactoside permease, one of the proteins encoded in the lac operon. This protein uses a proton-motive force to pump
lactose into the cell. The proton gradient is created by an electron transport complex in the membrane which is inhibited
by cyanide, reminiscent of the cytochrome C oxidase complex in oxphos.
Figure: Lactose Transport
- Glucose - Glucose can be transported into brush border cells lining the small intestine
powered by a sodium-motive symport transporter.
Figure: Glucose Transport

Examples of Transport: Protons
- Driven by oxidation - The proton gradient formed during aerobic oxidation and photosynthesis
in mitochondria and chloroplast, respectively, is paid for by free energy decreases associated with oxidation of organic molecules.
- Driven by ATP cleavage - As mentioned above, protons are transported into the the lumen
of the stomach by a K+-H+ ATPase.
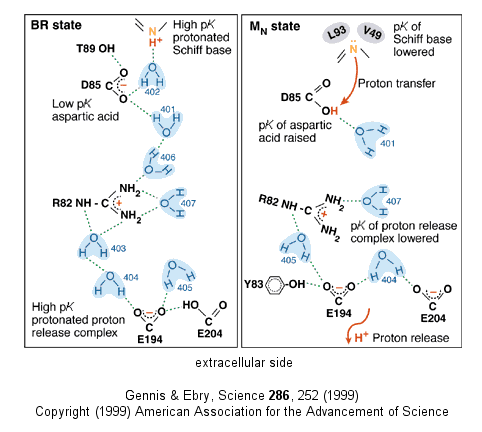
- Driven by light - Photosynthetic bacteria have a membrane protein called bacteriorhodopsin
which contains retinal, a conjugated polyene derived from beta-carotene. The retinal is covalently attached to the protein
through a Schiff base linkage to an epsilon amino group of Lys (much as pyridoxal phosphate is in PLP-dependent enzymes).
Bacteriorhodopsin is analogous to the visual pigment protein rhodopsin in retinal cells. Absorption of light by the retinal
induces a conformational changes in the all trans-retinal, which causes an associated conformational change in bacteriorhodopsin.
Various side chains and the protonated N of the Schiff base change their relative positions with respect to each other, which
leads to changes in protonation states of the side chains, to further conformational changes and ultimately vectorial
discharge of protons through the membrane.
Figure: BACTERIORHODOPSIN AND PROTON TRANSPORT
Figure: A NEW
VERSION SHOWING PROTON TRANSFER IN BACTERIORHODOPSIN
Examples of Other "Transporter" Powered by ATP
- Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) - This is a member of a family
of an ATP-Binding Cassette or ABC transporter proteins. The membrane protein has 12 transmembrane
helices. In contrast to other ion transporters which transport a discrete number of ions (3 sodium and 2 potassium ions,
for example), this changes conformation to form an open pore through which chloride ions flow. This protein is defective
in Cystic Fibrosis.
- Multidrug Resistance Transporter - MDR - This is another example of an ATP-Binding Cassette
or ABC transporter. It acts in a somewhat promiscuous fashion in pumping nonpolar toxic molecules out of the cell.
This would seem quite beneficial to the organism, unless the toxic molecule is a chemotherapeutic drug used to kill a tumor
cell.
- Phospholipid Flippase - This is a member of the P-Type ATPase family which instead
of moving ions across the membrane flips amino lipids (like PE) across leaflets in the bilayer. In an early chapter
we noted that flip-flop diffusion in liposomes was slow compared to that in cells, suggesting that the flip-flop diffusion was catalyzed in the cell.
Catalysis requires ATP cleavage and produces two conformations of the protein. During the conformational change of the
protein, a phospholipid appears to bind to the protein and is flipped to the other side of the membrane.
Experimental
Study of Flipping: Labeling new PL ; Assay for Flip-flop Diffusion
Drugs and Diffusion: Part 2
As mentioned earlier, one of the biggest problems in medical drug development is the productions of drugs
that can diffuse across the cell membrane. This requires that the drug be sufficiently nonpolar while at the same time
being sufficiently polar to have reasonable aqueous solubility, allowing blood transport. Another approach to getting
drugs across the membrane is to modify them to bind to transporters whose normal function is to move solutes against a concentration
gradient across a lipid bilyaer. The extent of modification of the drug depends on how close the structure of the drug
is in comparison to the normal ligand for the transporter. This approach has been used by the biotech company XenoPort, to develop drugs that can be more readily absorbed by the small intestine, which has many active transporters designed to
move nutrients into cells.
|

