We have just seen how we can transduce the chemical potential energy stored in carbohydrates into chemical
potential energy of ATP - namely through coupling the energy released during the thermodynamically favored oxidation of carbon
molecules through intermediaries (high energy mixed anhydride in glycolysis or a proton gradient in aerobic metabolism) to
the thermodynamically uphill synthesis of ATP. There is a situation that occurs when we wish to actually reverse the entire
process and take CO2 + H2O to carbohydrate + O2. This process is of course photosynthesis
which occurs in plants and certain photosynthetic bacteria and algae. Given that this process must by nature be an uphill
thermodynamic battle, let us consider the major requirements that must be in place for this process to occur:
- An "amazing" oxidizing agent must be formed which can take water and oxidize it to dioxygen. We
know that redox reactions occur in the direction of stronger to weaker oxidizing agent (just as acid base reactions are thermodynamically
favored in the direction of strong to weak acid). Somehow we must generate a stronger oxidizing agent than dioxygen, which
often has the most positive standard reduction potential in tables.
- Plants must have high concentrations of a reducing agent for the reductive biosynthesis of glucose
from CO2. The reducing agent used for most biosynthetic reactions in nature is NADPH, which differs from NADH only
by the addition of a phosphate to the ribose ring. This phosphate differentiates the pool of nucleotides in the cells used
for reductive biosynthesis (NADPH/NADP+) from those used for oxidative catabolism (NADH/NAD+)
- Finally, plants need an abundant source of ATP which will be required for reductive biosynthesis.
We will discuss only the light reaction of photosynthesis which produces these three types of molecules.
The dark reaction , which as the name implies can occur in the dark, involves that actual fixation of carbon dioxide
into carbohydrate using the ATP and NADPH produced in the light reaction.
THE LIGHT REACTION
Obviously, the energy to power the light reactions comes directly from sunlight. Clue two is that plants
have an organelle that animal cells don't - the chloroplast. Its structure is in many ways similar to a mitochondria in that
it has internal membranes (thylacoid membranes) surrounding enclosed compartments.
Plants have many pigments (chlorophyll, phycoerthryins, carotenoids, etc.) whose absorption spectra overlap
that of the solar spectra. The main pigment, chlorophyll, has a protophorphryin IX ring (same as in heme groups) with Mg instead
of Fe. When the chlorophyll absorbs light, the excited electrons must relax eventually to their ground state. It can do this
by either radiative or nonradiative decay. In radiative decay, a photon of lower energy is emitted (after some energy has
already been lost by vibrational transitions) in a process of either fluorescence or phosphorescence. In nonradiative
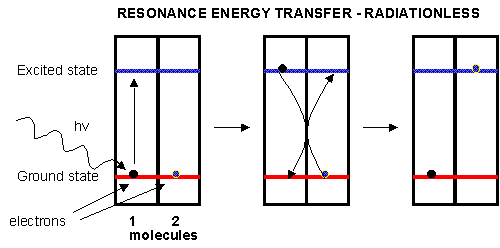
decay, the energy of an excited electron can be transferred to another similar molecule (in this case other chlorophyll molecules)
in a process which excites the energy of an electron in the second molecule to the same excited state. (It is as if a photon
is released by the first excited molecule which then is absorbed by an electron in a second molecule to excite it to the same
exited state, although the excitation occurs without photon production). In this fashion, energy is transferred from
one chlorophyll to another. This type of energy transfer is called resonance energy transfer or exciton transfer.
Figure: resonance energy transfer
One type of chlorophyll has slightly different characteristics, however. Because of its unique environment,
the energy level of the excited state electron is lower than in the rest of the chlorophyll molecules, in much the same way
that pKa's of amino acid side chains differ with environment, and the standard reduction potential of FADs that are tightly
bound to enzymes differ due to the different environment of FAD/FADH2 These unique chlorophyll centers are
called reaction centers.
Figure: reaction centers
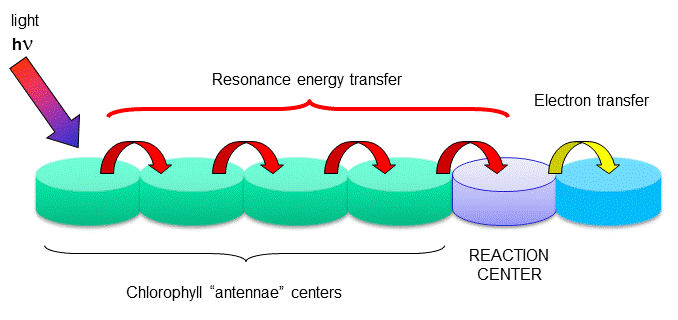
The rest of the chlorophyll molecules act as antennas which transfer energy to the reaction centers. An
electron in an adjacent excited state chlorophyll (which is at a higher level than the excited state energy of the reaction
center) can then be transferred to this lower energy state level in the reaction center, in a process which forms a positively
charged ion from the first excited state molecule and an anion from the recipient. This process of energy transfer is called
electron transfer.
Figure: electron transfer
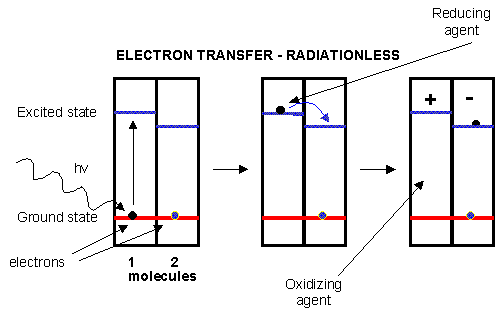
Photoexcitation of the non-reaction center chlorophyll turns that molecule into a good reducing agent,
which transfers its electron to the excited state level of the reaction center chlorophyll. If you count both step together,
the non-reaction center chlorophyll gets "photooxidized", in the process producing the "amazing" oxidizing agent which is
the positively charged chlorophyll derivative. The extra electron passed onto the second molecule will eventually be passed
on to NADP+ to produce NADPH.
Figure: THE LIGHT REACTIONS OF PHOTOSYNTHESIS
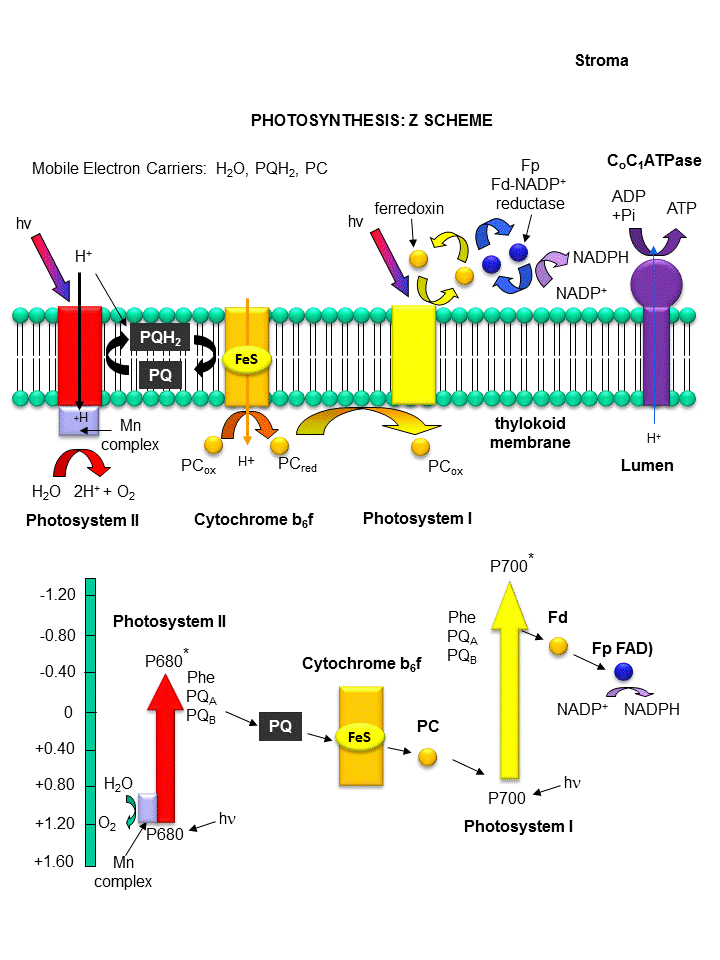
Photosystem II:
Recently the crystal structure of PSII from a photosynthetic cyanobacterium was determined. It consists
of 17 polypeptide subunits with metal and pigment cofactors and over 45,000 atoms. (Zouni, Nature, 409, 739, 2001).
Of particular interest is the P680 chlorophyll reaction center, which consists of four monomeric chlorphylls adjacent to a
cationic Tyr-D side chain which destabilizes the chlorophyll molecules. When H2O gets oxidized to form dioxygen,
4 electrons must be remove by photoactivated P680. In PSII, this process occurs in 4, single electron steps, with the
electrons first being transferred to a Mn4 cluster cofactor (of composition Mn4Ca1Cl1-2(HCO3)y.
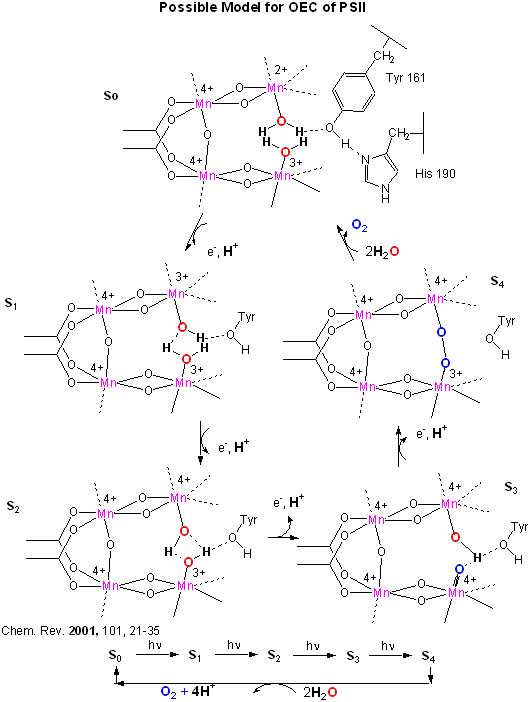
This inorganic Mn cluster is often called OEC (oxygen evolving complex) or WOC (water oxidizing complex). The
electrons passed through the Mn complex are delivered to P680 by a photoactive Tyr free radical (Tyr Z). The actual
structure of the OEC could not be resolved, but other structural and spectroscopic data support the structure below (Chem.
Rev., 2001, 101, 21-35), which also shows a possible mechanism for electron and proton transfer from water
to form dioxygen. 5 discrete intermediates of the OEC, S0-S4, are suggested from the experimental
data (Kok cycle). These were postulated from experiments in which spinach chloroplast were illuminated with short
light pulses. A pattern of dioxygen release was noted that repeated after 4 flashes. Ultimately, light absorption
by P680 forms excited state P680* which donates an electron to pheophytin (which passes them to quinones) to form P680+,
which receives electrons from the OEC, specifically the TyrZ radical.
Figure: OEC - Mechanism of Water Oxidation
Investigators have made non-peptide mimetics of superoxide dismutase to facilitate therapeutic removal
of excess superoxide formed in brain and heart tissue. These may arise after an oxidative burst from
reperfusion of these tissues after heart or brain attacks. Likewise, scientist are trying to build synthetic PSII-OEC
complexes which could be used to form dioxygen or hydrogen gas for fuels.
Plant Protection
Plants have evolved a great ability to absorb light over the entire visible range of the spectra. Can they absorb
to much energy. The answer is yes, so plants have developed many ways to protect themselves. IF too much light is absorbed,
the pH gradient developed across the thylacoid membranes becomes greater. This is sensed by a protein, PsbS, and through
subsequent conformational changes transmitted through the light-harvesting antennae, the excess light energy is dissipated
as thermal energy. Mutants lacking PsbS showed decreased seed yield, a sign that it became less adaptable under conditions
of stress (such as exposure to rapidly fluctuating light levels. Molecules called xanthophylls (synthesized from carotenes
- vit A precursors) such as zeaxanthin are also important in excess energy dissipation. These molecules appear to cause
excited state chlorophyll (a singlet like excited state dioxygen) to become deexcited. Without the xanthophylls, the
excited state chlorophyll could deexcite by transfer of energy to ground state triplet dioxygen, promoting it to the singlet,
reactive state, which through electron acquisition, could also be converted to superoxide. These reactive oxygen species
(ROS) can lead to oxidative damage to proteins, lipids and nucleic acids, alteration in gene transcription, and even programmed
cell death. Carotenoids can also acts as ROS scavengers. Hence both heat dissipation and inhibition of formation
of ROS (by such molecules as vitamin E) are both mechanism of defense of excessive solar energy
Given that both plants and animals must be protected from ROS, antioxidant molecules made by plants may prove to protect
humans from diseases such as cancer, cardiovascular disease, and general inflammatory diseases, all of which have been shown
to involve oxidative damage to biological molecules. Humans, who can't synthesize the variety and amounts of antioxidants
that are found in plants, appear to be healthy when they consume large amounts of plant products. These phytomolecule
also have other properties, including regulation of gene transcription which can also have a significant effect on disease
propensity.
Production of Hydrogen: Hydrogenases
Our world desperately needs an energy efficient way to produce H2 for energy production without producing waste
pollutants. Catalytic cracking of molecules and newly developed fuel cells offer two possibilities. Wouldn't it be great
if water could be used for H2 production (without the use of electrolysis) or expensive metal catalysts?
Nature may show the way. Simple bacteria (even E. Coli found in our GI system) can use simple metals like iron to produce
H2 from water (which can be used by the organism as an energy source). The enzymes that catalyze hydrogen
production are hydrogenases.
Crystal structures of hydrogenases show them to be unique among metal-containing enzymes. They contain two metals bonded
to each other. The metal centers can either be both iron or one each of iron and nickel. The ligands interacting
with the metals are two classical metabolic poisons, carbon monoxide and cyanide. Passages for flow of electron and
H2 connect the buried metals and the remaining enzymes. The metals are also bound to sulfhydryl groups of cysteine
side chains. It appears that two electrons are added to a single proton making a hydride anion which accepts a proton
to form H2. In the two Fe hydrogenases, the geometry of the coordinating ligands distorts the bond
between the two iron centers, leading to irons with different oxidation numbers. Electrons appear to flow from one center
to the other, as does carbon monoxide as well. Ultimately, hydrogenases or small inorganic mimetics of the active site
could be coated on electrons and used to general H2 when placed in water in electrolytic experiments.
Web Links:

