|
Glycoprotein Biosynthesis
Carbohydrates are added to proteins in a very complicated process which involves two organelles, the endoplasmic
reticulum and the Golgi apparatus. CHO addition to proteins occurs both co- and post-translationally. The RNA
coding the protein sequence enters the cytoplasm where it binds to ribosomes (large RNA-protein complexes) which are the site
for protein synthesis. Cytoplasmic proteins are synthesized on free ribosomes, but for future glycoproteins, the ribosomes
bind to an elongated, extensive organelle in the cell called the endoplasmic reticulum (ER).
Figure: ribosomes bind to an elongated, extensive organelle in the cell called the
endoplasmic reticulum (ER)

(from web, searching for reference)
The nascent protein chain enters the lumen of the ER and a core oligosaccharide is added to the protein.
Further additions and removal (trimming and processing) of monosaccharides are preformed in the lumen until a final core mannose
structure has been added. This is demonstrated in the animation below. (The pink two-lobbed structure represents
the ribosome which is bound to the ER membrane. The newly synthesized protein is shown in the lumen side of the ER membrane.
That is where glycan transfer occurs.) The ER lumen contains high concentrations of molecular chaperones to assist protein
folding.
Now additional carbohydrate modifications (post-translational) are made as the protein moves from the
lumen of the ER (probably by a budding process) to another series of stacked, pancake-like organelles called the Golgi apparatus.
Here terminal carbohydrate modification is completed. The Golgi does not contain molecular chaperons since protein
folding is complete when the proteins arrive. Rather they have high concentrations of membrane bound enzymes, including
glycosidases, and glycosyltransferases.
Glyprotein Function
The role of CHO in glycoprotein structure/function is slowly being determined. The most important seems
to involve their role in directing proper folding of proteins in the ER which accounts for the observations that glycan addition
to proteins in the ER is a cotranslational event. When inhibitors of ER glycosylation are added to cells, protein misfolding
and aggregation are observed. The extent of misfolding depends on the particular protein and particular glycosylation
sites with the protein. The polar CHO residues help promote solubility of folding intermediates, similar to the effects
of many chaperone proteins.
The glycan moieties of the folding glycoprotein also lead to binding of the protein to lectins in the
ER which serve as molecular chaperones. The most studied of these chaperones are involved in the calnexin-calreticulin
cycle, and facilitate correct disulfide bond formation in the protein. After two glucose residues are removed by glucosidase I and II, the monoglucosylated protein
binds to calnexin (CNX) and/or calreticulin (CRT), two homologous ER lectins specific for monoglucosylated proteins. Once bound, another protein, ERp57, a molecular chaperone with
a disulfide bond (shown in diagram) interacts with the protein. This protein has protein disulfide isomerase activity.
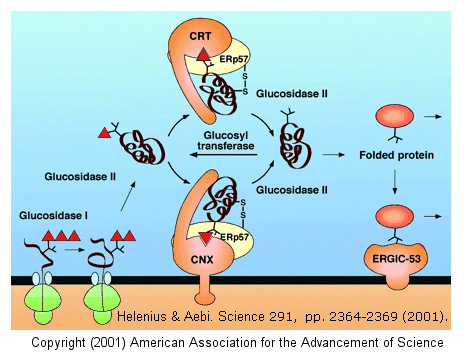
If a glycoprotein has not folded completely, it is recognized by a glycoprotein glucosyltransferase, which
adds a glucose to it. This then promotes reentry into the calnexin/calreticulin cycle.
Ideally, unfolded or misfolded proteins would be targeted from degradation and elimination from cells.
The ER has evolved a system to accomplish this. Since folding occurs in the ER, to prevent misfolding and
aggregation, the ER also contains chaperones and folding catalysts. Stress (such as through heat shock) stimulates ER
chaperone activity. As a final defense mechanism, unfolded or aberrantly-folded proteins are degraded by the cytoplasmic proteasome
complex. Nonnative forms of some proteins that "escape" this surveillance system can accumulate and result in disease
(for example neurodegenerative diseases like Alzheimers and Parkinson's disease.
Glycobiology
Our understanding of the synthesis and structure of glycan portions of glycoproteins has lagged behind
our understanding of protein and nucleic acid structure and synthesis. Several reasons account for this:
- carbohydrates are much more complex with more functional groups per carbon and with a much larger number
of stereocenters, making chemical synthesis and structure determination more difficult.
- carbohydrate chain synthesis is not directed by a template as is the synthesis of DNA, RNA, and proteins
- synthesis is spread over two different organelles, and which allows great heterogeneity in main chain
and branch chain synthesis, which provides heterogeneous samples for analysis
New techniques in analysis and synthesis of carbohydrate analogs and inhibitors of enzymes involved in
CHO synthesis and degradation, as well as in genetic manipulations of gene for these enzymes, is revolutionizing our understanding
of the function of carbohydrate groups on lipids and proteins. On a more practical note, new methods to synthesize glycoproteins
using recombinant DNA technology have been developed that allow synthesis of therapeutic human glycoproteins in yeast.
Although they share many of the same synthetic steps, yeast glycoproteins are enriched n the high-mannose type, making them
targets of the human immune system. Human and yeast glycoproteins synthesis produce the same mannose core in the ER
(as illustrated in the animation above). However, differences in synthesis occurs in the Golgi. Human Golgi contain
a-mannosidases I and II, which remove all but 3 Man residues from the final
product. In yeast, however, these mannosidases appear to be missing so more Man residues are added (as many as 100).
Human proteins made in yeast, therefore, contain many Man residue, which are recognized by the human immune system.
So address these issues, Hamilton et al produced mutants of the yeast Pichia pastoris that localized glycoprotein synthesis
proteins for mannosidase I and II, as well as other human glycoprotein synthesis genes, to the correct intracellular location
while inactivating normal yeast gene, resulting in the production of human glycoproteins with the correct CHO structure in
yeast. This may prove to have widespread use in the production of therapeutic human proteins.
|

