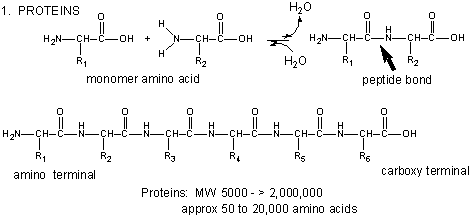
Amino acids form polymers through a nucleophilic attack by the amino group of an amino acid
at the electrophilic carbonyl carbon of the carboxyl group of another amino acid. The carboxyl group of the amino acid must
first be activated to provide a better leaving group than OH-. (We will discuss this activation by ATP latter
in the course.) The resulting link between the amino acids is an amide link which biochemists call a peptide bond. In this
reaction, water is released. In a reverse reaction, the peptide bond can be cleaved by water (hydrolysis).
When two amino acids link together to form an amide link, the resulting structure is called a dipeptide.
LIkewise, we can have tripeptides, tetrapeptides, and other polypeptides. At some point, when the structure is long
enough, it is called a protein. There are many different ways to represent the structure of a polypeptide or protein.
each showing differing amounts of information. .
Figure: Different Representations of a Polypeptide (heptapeptide)
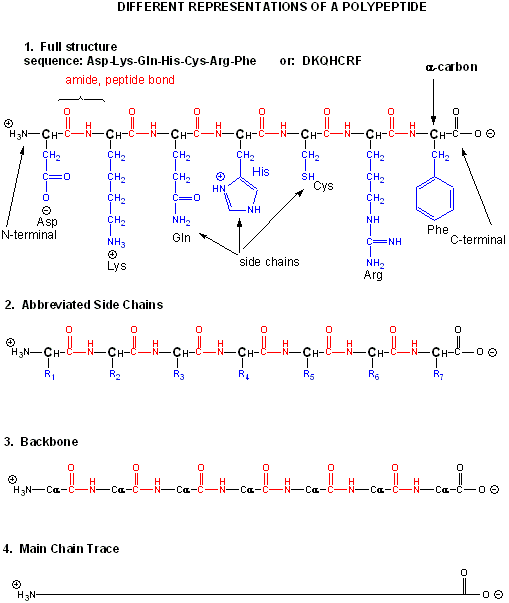
Figure: Amino Acids React to Form Proteins
Proteins are polymers of twenty naturally occurring amino acids. In contrast, nucleic acids are polymers
of just 4 different monomeric nucleotides. Both the sequence of a protein and it's total length differentiate one protein
from another. Just for an octapeptide, there are over 25 billion different possible arrangement of amino acids. Compare this
to just 65536 different oligonucleotides of 8 monomeric units (8mer). Hence the diversity of possible proteins is enormous.
Stereochemistry
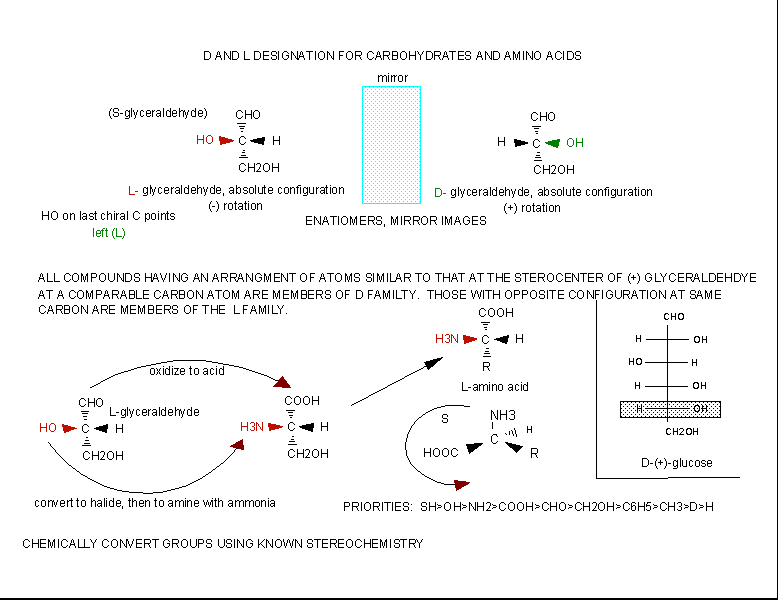
The amino acids are all chiral, with the exception of glycine, whose side chain is H. As with lipids,
biochemists use the L and D nomenclature. All naturally occuring proteins from all living organisms consist of L amino acids.
The absolute stereochemistry is related to L-glyceraldehyde, as was the case for triacylglycerides and phospholipids. Most
naturally occurring chiral amino acids are S, with the exception of cysteine. As the diagram below shows, the absolute configuration
of the amino acids can be shown with the H pointed to the rear, the COOH groups pointing out to the left, the R group to the
right, and the NH3 group upwards. You can remember this with the anagram CORN.
Figure: Stereochemistry of Amino Acids.
Why do Biochemistry still use D and L for sugars and amino acids? This explanation (taken from the
link below) seems reasonable.
"In addition, however, chemists often need to define a configuration unambiguously in the absence of any reference compound,
and for this purpose the alternative (R,S) system is ideal, as it uses priority rules to specify configurations. These
rules sometimes lead to absurd results when they are applied to biochemical molecules. For example, as we have seen, all of
the common amino acids are L, because they all have exactly the same structure, including the
position of the R group if we just write the R group as R. However, they do not all have the same configuration in the (R,S)
system: L-cysteine is also (R)-cysteine, but all the other L-amino acids are (S),
but this just reflects the human decision to give a sulphur atom higher priority than a carbon atom, and does not reflect
a real difference in configuration. Worse problems can sometimes arise in substitution reactions: sometimes inversion of configuration
can result in no change in the (R) or (S) prefix; and sometimes retention of configuration can result
in a change of prefix.
It follows that it is not just conservatism or failure to understand the (R,S) system that causes biochemists to
continue with D and L: it is just that the DL system fulfils their
needs much better. As mentioned, chemists also use D and L when they are appropriate to their
needs. The explanation
given above of why the (R,S) system is little used in biochemistry is thus almost the exact opposite of reality. This system
is actually the only practical way of unambiguously representing the stereochemistry of complicated molecules with several
asymmetric centres, but it is inconvenient with regular series of molecules like amino acids and simple sugars. "
If I told you to draw the correct stereochemistry of a molecule with 1 chiral C (S isomer for example)
and I gave you the substituents, you could do so easily following the R, S priority rules you learned in organic. However,
how would you draw the correct isomer for the L isomer of the amino acid alanine? You couldn't do it without
prior knowledge of the absolute configuration of the related molecule, L glyceraldehyde, or unless you remembered the anagram
CORN. This disadvantage, however, is more than made up for by the fact that different L amino acids with the same absolute
stereochemistry, might be labeled R or S , which makes this nomenclature unappealing to biochemists.
Charge Characteristics
Monomeric amino acids have an alpha amino group and a carboxyl group, both of which may be protonated
or deprotonated, and a R group, some of which may be protonated or deprotonated. When protonated, the amino group has a +1
charge, and the carboxyl group a 0 charge. When deprotonated the amino group has no charge, while the carboxyl group has a
-1 charge. The R groups which can be protonated/deprotonated include Lys, Arg and His, which have a + 1 charge when
protonated, and Glu and Asp (carboxylic acids), Tyr and Ser (alcohols) and Cys (thiol), which have 0 charge when protonated.
Of course, when the amino acids are linked by peptide bonds (amide link), the alpha N and the carboxyl C are in an amide link,
and are not charged. However, the amino group of the N -terminal amino acid and the carboxyl group of the C-terminal amino
acid of a protein may be charged. The Henderson Hasselbach equation gives us a way to determine the charge state of any ionizable
group knowing the pKa of the group. Write each functional group capable of being deprotonated as an acid, HA, and the deprotonated
form as A. The charge of HA and A will be determined by the functional group. The Ka for the reaction is:
Ka = [H3O+][A]/[HA]. or
[H3O+] = Ka[HA]/[A].
- log [H3O+] = -log Ka + log [A]/[HA]
or pH = pKa + log [A]/[HA]
This is the (in)famous Henderson-Hasselbach (HH) equation.
The properties of a protein will be determined partly by whether the side chain functional groups, the
N terminal, and the C terminal are charged or not. The HH equation tells us that this will depend on the pH and the pKa of
the functional group.
- If the pH is 2 units below the pKa, the HH equation becomes, -2 = log A/HA, or .01 = A/HA. This means
that the functional group will be about 99% protonated (with either 0 or +1 charge, depending of the functional group).
- If the pH is 2 units above the pKa, the HH equation becomes 2 = log A/HA, or 100 = A/HA. Therefore the
functional group will be 99% deprotonated.
- If the pH = pka, the HH equation becomes 0 = log A/HA, or 1 = A/HA. Therefore the functional group will
be 50% deprotonated
From these simple examples, we have derived the +2 rule. This rule is used to quickly determine
protonation, and hence charge state, and is extremely important to know (and easy to derive). Titration curves
for Gly (no ionizable) side chain, Glu (carboxlic acid side chain) and Lys (amine side chain) are shown below. You should
be able to associate various sections of these curves with titration of specific ionizable groups in the amino acids.
Figure: Titration curves for Gly, Glu, and Lys
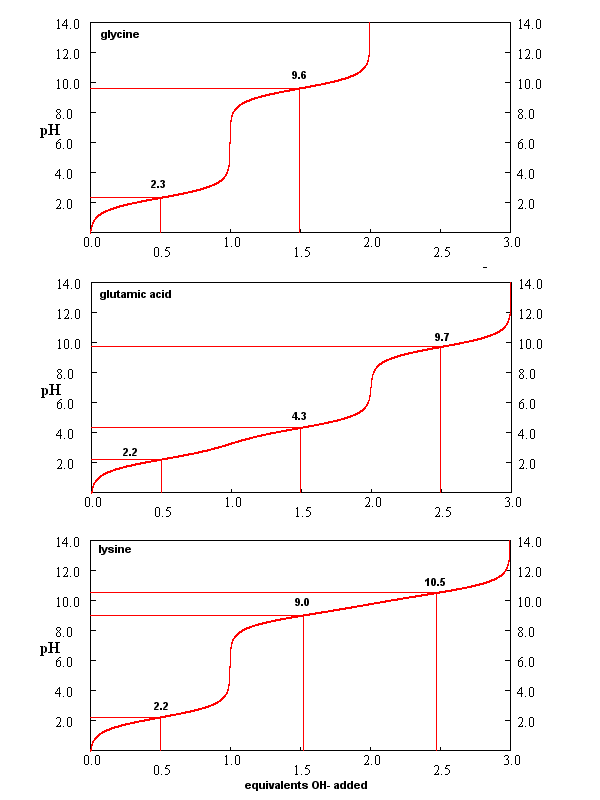
Isoelectric Point
What happens if you have many ionizable groups in a single molecule, as is the case with a polypeptide
or protein. Consider a protein. At a pH of 2, all these groups would be protonated, and the overall charge of
the protein would be positive. (Remember, when carboxylic acid side chains are protonated, there net charge is 0.) As the
pH is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At high pH,
all the ionizable groups will become deprotonated in the strong base, and the overall charge of the protein will be negative.
At some pH, then, the net charge will be 0. This pH is called the isoelectric point (pI). The pI can
be determined by averaging the pKa values of the two groups which are closest to and straddle the pI. One of the online problems
will address this in more detail
Remember that pKa is really a measure of the equilibrium constant for the reaction. And of course, you
remember that DGo = -RT ln Keq. Therefore, pKa is independent
of concentration, and depends only on the intrinsic stability of reactants with respect to the products. This is true
only AT A GIVEN SET OF CONDITIONS, SUCH AS T, P, AND SOLVENT CONDITIONS.
Consider, for example acetic acid, which in aqueous solution has a pKa of about 4.7. It is a weak acid,
which dissociates only slightly to form H+ (in water the hydronium ion, H3O+, is formed)
and acetate (Ac-). These ions are moderately stable in water, but reassociate readily to form the starting product.
The pKa of acetic acid in 80% ethanol is 6.87. This can be accounted for by the decrease in stability of the charged products
which are less shielded from each other by the less polar ethanol. Ethanol has a lower dielectric constant than does water.
The pKa increases to 10.32 in 100% ethanol, and to a whopping 130 in air!
Chemical Reactivity of Amino Acid Side Chains
You should be able to identify which side chains contain H bond donors and acceptors. Likewise, some are
acids and bases. You should be familiar with the approximate pKa's of the side chains, and the N and C terminal groups. Three
of the amino acid side chains (Trp, Tyr, and Phe) contribute significantly to the UV absorption of a protein at 280 nm.
This section will dealing predominantly with the chemical reactivity of the side chains, which is important in understanding
the properties of the proteins. Many of the side chains are nucleophiles. Nucleophilicity is a measure of
how rapidly molecules with lone pairs of electrons
can react in nucleophilic substitution reactions. It correlates with basicity, which measures the extent to which a molecule with lone pairs can react with an acid (Bronsted
or Lewis). The properties of the atom which holds the lone pair are important in determining both nucleophilicity and basicity.
In both cases, the atom must be willing to share its unbonded electron pair. If the atoms holding the nonbonded pair is more
electronegative, it will be less likely to share its electrons, and that molecule will be a poorer nucleophile (nu:) and weaker
base. Using these ideas, it should be clear that RNH2 is a better nucelophile than ROH, OH- is a better than
H2O and RSH is a better than H2O. In the latter case, S is bigger and its electron cloud is more polarizable
- hence it is more reactive. The important side chain nucleophiles (in order from most to least nucleophilic) are Cys
(RSH, pKa 8.5-9.5), His (pKa 6-7), Lys (pKa 10.5) and Ser (ROH, pKa 13).
An understanding of the chemical reactivity of the various R group side chains of the amino acids in a
protein is important since chemical reagents that react specifically with a given amino acid side chain can be used
to:
- to identify the presence of the amino acids in unknown proteins or
- to determine if a given amino acid is critical for the structure or function of the protein. For
example, if a reagent that covalently interacts with only Lys is found to inhibit the function of the protein, a lysine
might be considered to be important in the catalytic activity of the protein.
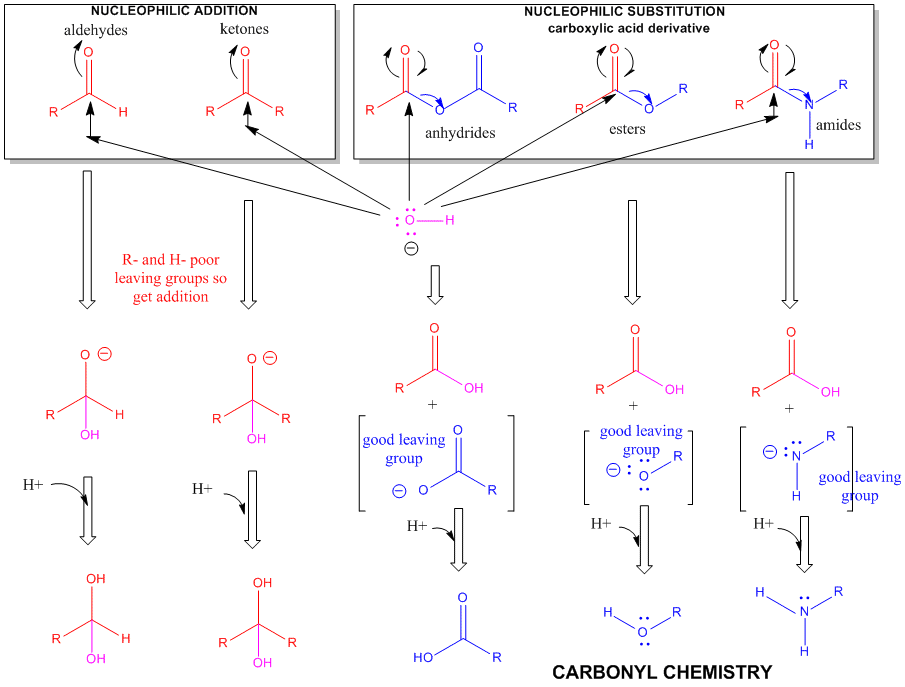
Figure: A REVIEW SUMMARY OF THE CHEMISTRY OF ALDEHYDES, KETONES, AND CARBOXYLIC
ACID DERIVATIVES
1. Ser: Generally no more reactive than ethanol. It is a potent nucleophile in
a certain class of proteins (proteases, for example) when it is deprotonated.
2. Lys: (or N-terminal RNH2) This is a potent nu: only when deprotonated.
Figure: LYSINE REACTIONS 1
- reacts with anhydride in a nucleophilic substitution reaction (acylation).
- reacts reversibly with methylmaleic anhydride (also called citraconic anhydride) in a nucleophilic substitution
reaction.
- reacts with high specificity and yield toward ethylacetimidate in a nucleophilic substitution reaction
(ethylacetimidate is like ethylacetate only with a imido group replacing the carbonyl oxygen). Ethanol leaves as the
amidino group forms. (has two N -i.e. din - attached to the C)
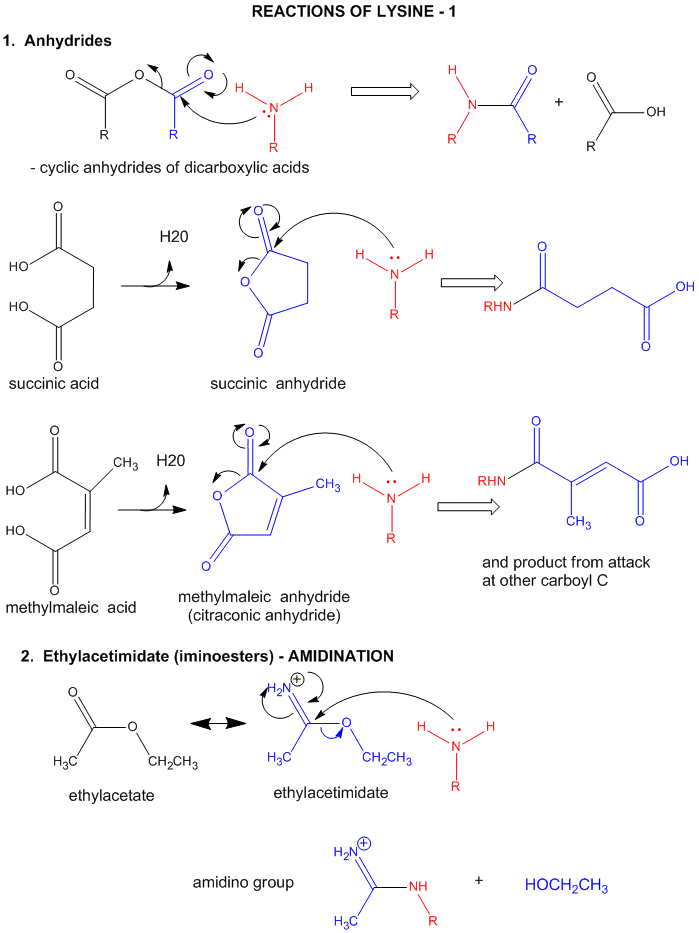
Figure: LYSINE REACTIONS 2
- reacts with O-methylisourea in a nucleophilic substitution reaction. with the expulsion of methanol to
form a guanidino group (has 3 N attached to
C, nidi)
- reacts with fluorodintirobenzene (FDNB or Sanger's reagent) or trinitrobenzenesulfonate (TNBS, as we
saw with the reaction with phosphatidylethanolamine) in a nucleophilic aromatic substitution reaction to form 2,4-DNP-lysine
or TNB-lysine.
- reacts with Dimethylaminonapthelenesulfonylchloride (Dansyl Chloride) in a nucleophilic substitution
reaction.
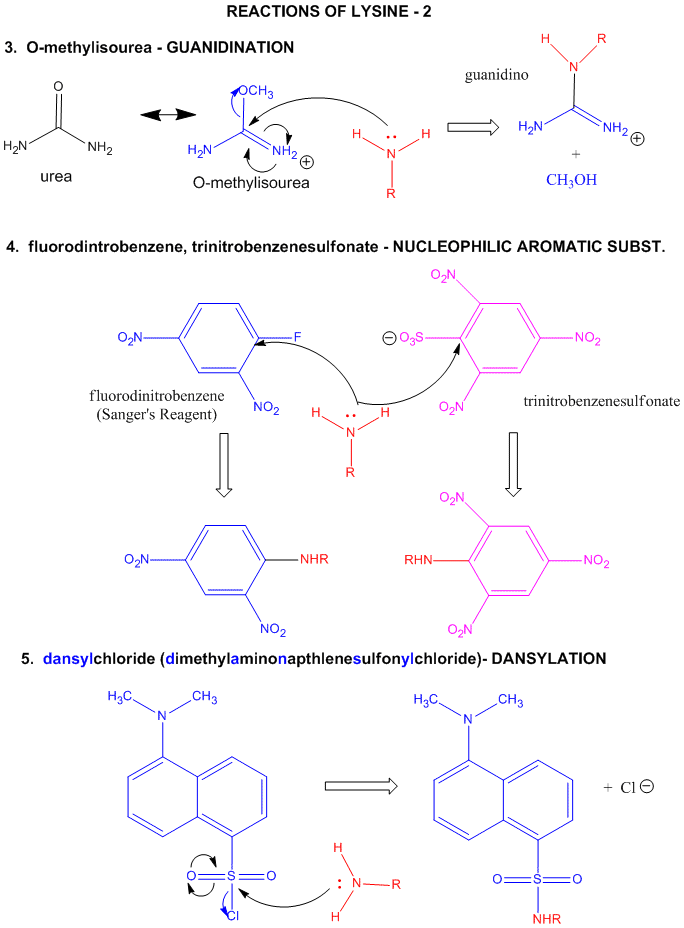
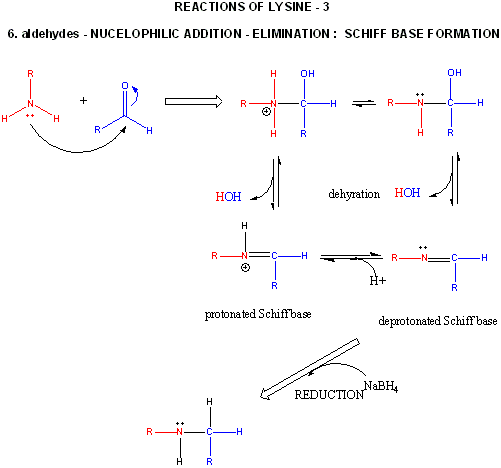
Figure: LYSINE REACTIONS 3
- reacts with high specificity toward aldehydes to form imines (Schiff bases), which can be reduced with
sodium borohydride or cyanoborohydride to form a secondary amine.
3. Cys: a potent nucleophile, which is often linked to another Cys to form a covalent
disulfide bond.
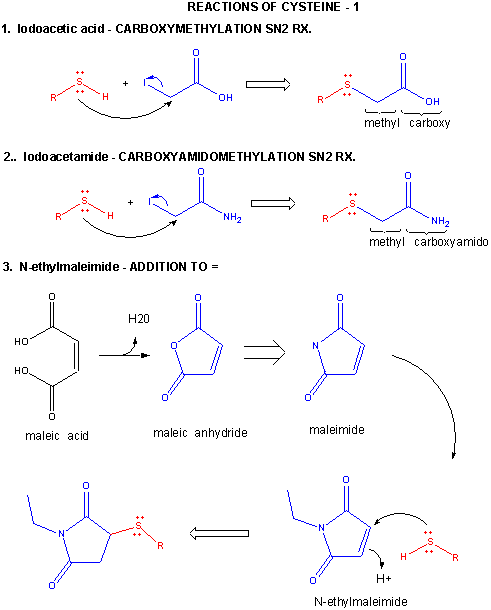
Figure: CYSTEINE REACTIONS 1
- reacts with iodoacetic acid in an SN2 rx., adding a carboxymethyl group to the S.
- reacts with iodoacetamide in an SN2 rx, adding a carboxyamidomethyl group to S.
- reacts with N-ethylmaleimide in an addition rx. to the double bond
Figuire: a quick review of sulfur
redox chemistry
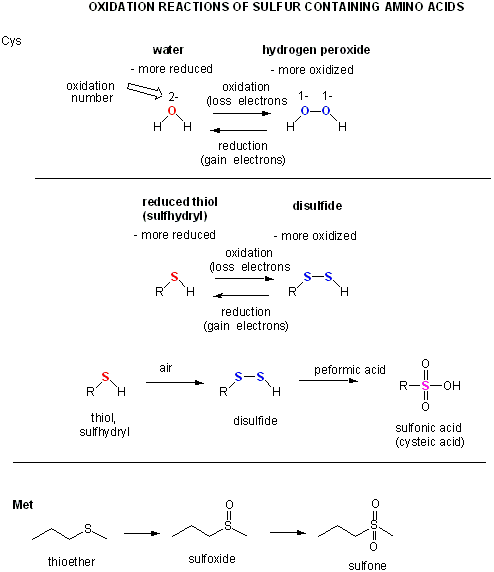
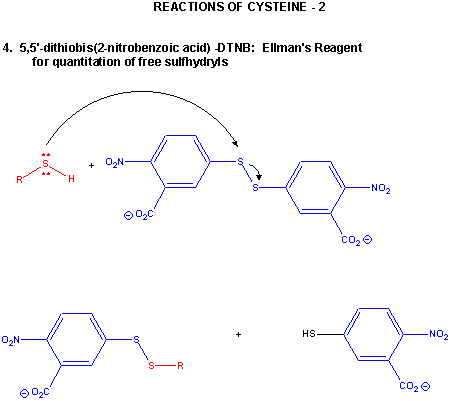
Figure: CYSTEINE REACTIONS 2
- reacts with R'-S-S-R'', a disulfide, in a disulfide interchange reaction, to form R-S-S-R'
- reacts with oxidizing agents like HCOOOH, performic acid, to form cysteic acid.
- reacts with 5,5-Dithiobis (2-nitrobenzoic acid) (DTNB or Ellman's reagent) in a RSH displacement reaction
in which DTNB is cleaved and the 2-nitro-5-thiobenzoic acid anion, which absorbs at 412 nm, is released. Used to quantitate
total RSH in a protein
Cystine - Disulfides:
Two cysteine side chains can covalently interact in a protein to produce a disulfide. Just as HOOH (hydrogen
peroxide) is more oxidized than HOH (O in H2O2 has oxidation number of 1- while the O in H2O
has an oxidation number of 2-) , RSSR is the oxidized form and RSH is the reduced form of thiols.
Figure: DISULFIDE - CYSTINE - REACTIONS
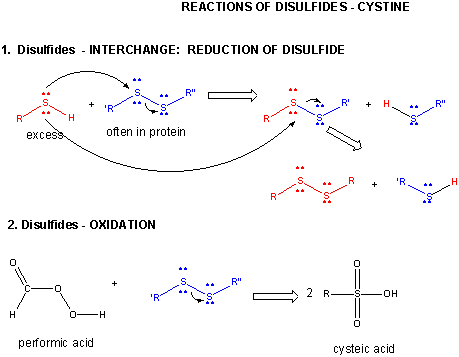
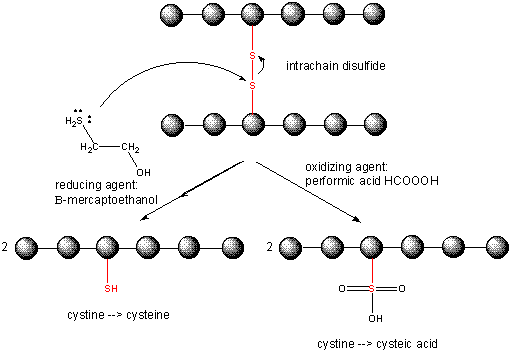
When a protein folds, two Cys side chains might approach each other, and form an intrachain disulfide
bond. Likewise, two Cys side chains on separate proteins might approach each other and form an interchain disulfide. Such
disulfides must be cleaved, and the chains separated before analyzing the sequence of the protein. The disulfide in protein
can be cleaved by reducing agents such as beta-mercaptoethanol or oxidizing agents, which further oxidizes the disulfide to
separate cysteic acids. The inside of cells are maintained in a reduced environment by the presence of many "reducing" agents,
such as the tripeptide g-glu-cys-gly (glutathione). Hence intracellular proteins
usually do not contain disulfides, which are abundant in extracellular proteins (such as those found in blood).
Figure: CLEAVING DISULFIDE BONDS IN PROTEINS
4. Histidine: one of the stronges bases at physiological pH's.
A secondary amine (which through electron release might be expected to be a stronger nucleophile
than a primary amine, although this effect is usually canceled by steric hindrance of the N by the two attached C's).
However, in His, the steric effects are minimized since the 2Cs are restrained by the ring. With a pKa of about 6.5, this
amino acid is one of the strongest available bases at physiological pH (7.0). Hence, it can often cross-react with many of
the reagents used to modify Lys side chains. His reacts with reasonably high selectivity with diethyl pyrocarbonate.
Figure: REACTIONS OF HISTIDINE
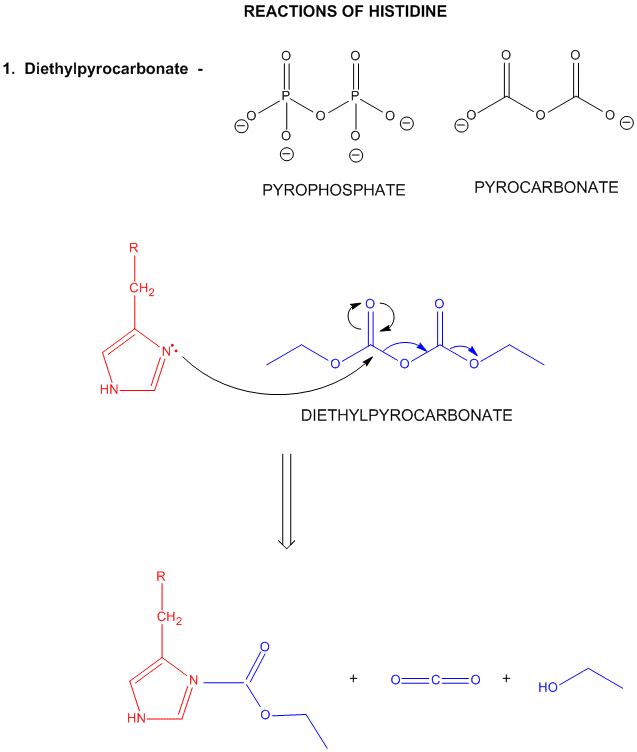
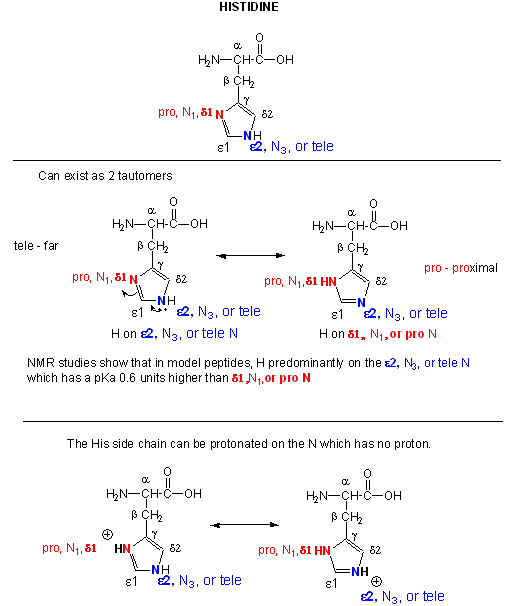
Figure: Where is the H on His? Where is the Charge?
Amino acids in naturally occurring proteins are also subjected to chemical modification within cells.
These modifications alter the properties of the amino acid that is modified, which can alter the structure and function of
the protein. Most chemical modifications made to proteins within cells occur after the protein is synthesized in a process
called translation. The resulting chemical changes are termed post-translational modifications.
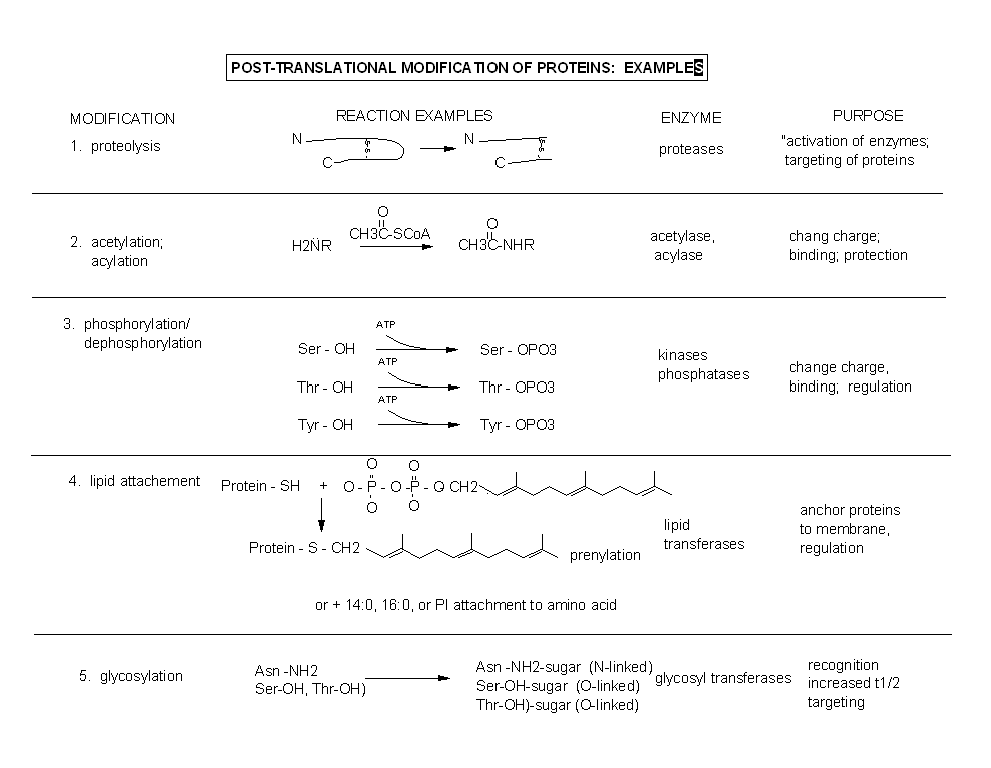
Figure: Post-translational modification of proteins