Making Liposomes
Micelles and liposomes form spontaneously - i.e. DG <
0. When water is added to dried phospholipids, multi-lamellar vesicles (MLVs) form, with water trapped
between the successive polar head groups of the multiple bilayers. Sonicating these will produce small unilamellar vesicles
(SUVs). If however, an aqueous detergent solution (such as b-D-octylglucopyranoside)
is added to a concentration greater than its CMC, mixed micelles of the phospholipid and detergent result. By slowly dialyzing
away the detergent, which is selected on the basis of its high CMC, the phospholipids in the micelle aggregate to form a large
unilamellar vesicle (LUV). Various drugs may be encapsulated in the liposome by placing them in solution before the dialysis
step.
Why do single chain amphiphiles form micelles and double chain amphphiles form bilayers?
As the number of C in the alkyl chain increase, the Dm
for HC transferring into a micelle, or by analogy, for a single chain amphiphile entering a micelle, becomes more and more
negative. The following equation seems to apply to the transfer of a single chain amphiphile into a micelle:
Dmo = mo
(mic) - mo (aq) = + number - 709 NC
where NC is the number of carbon atoms in the chain. The first term depends on the nature of the head
group, while the second is independent of the head group. These + and - terms brings us back to the principle of opposing
forces we discussed when we looked at the intemolecular forces involved in micelle and bilayer formation. There are attractive
IMFs, including van der Waals interactions among the chains and dipole-ion, and H-bond interactions with water and the head
groups. There are likewise repulsive interactions arising from steric hindrance with bulky heady groups and ion-ion repulsions.
Of course there are also entropic considerations. Let us now consider these factors as we explore what might happen
to a preformed micelle as we try to put more single chain amphiphiles (sca) into it. Clearly, as we increase the
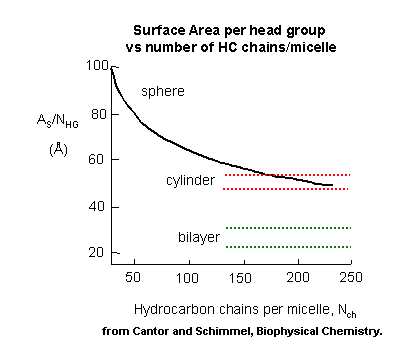
number of C's in the sca, the micelles would have a larger radius. For a given sca of fixed number of C's, once a spherical
micelle is formed, it can no longer retain its spherical shape if more sca's are added. Imagine increasing the
diameter of a spherical micelle 10x. A large part of the the inside would have no atoms or be filled with water, which
would not be favorable. Therefore, if the micelle is to grow, it can do so only by changing shape to something other
than a sphere. By squeezing a ball, one can imagine the the shape could distort to a circular cylinder. In this way
the acyl chains can still interact. The only problem is that head groups will now be closer than they were in the sphere.
Imagine in a sphere the head groups radiating in a perpendicular direction from the sphere surface. As the sphere is distorted
to a cylinder, the head groups would come closer together, and hence they will experience more steric interference.
If a cylinder can be formed, however, it could continue to grow as long as needed with no further compression required.
Image now that you further compressed the cylinder into a planar "bilayer" structure. The head groups would be even
closer and experience even more repulsion. This bilayer will not form since growth can occur in the cylindrical
phase without the added repulsion.
Now consider a double chain amphiphile (dca). In the case of a sca, the number (N) of head groups
(HG) = the number of acyl chains (CH). Hence the surface area per HG is equal to the area per HC. Or: As/N
HG = As/ N CH. For a dca, N HG = N CH/2, therefore As/N
HG = 2As/NCH. There is twice the surface area available per head group compared to that of the
sca. Therefore the dca can tolerate more compression. In fact, it can easily be compressed to a bilayer, which
as we saw, has much less As/HG. The cylindrical form actually has to much space per head group since water can
enter the structure. The extra closeness of head groups in the bilayer can be tolerated even more, since the Dmo for transfer of a dca into a micelle is 60% more negative than that of
a sca. The As/HG for closed vesicles differs only slightly from that of a truly planar bilayer since the vesicles
are so large compared to a micelle.
Once again, we have discovered that structure mediates function. We can account for the fact that sca
and dca form micelles and bilayers, respectively, by understanding the structure of the monomers!
Figure: Surface Area per Head Group vs no. of C in Amphiphile - Globular, Cylindrical,
Planar Forms.