Chemical Potential
We have studied the intermolecular forces involved when single chain amphiphiles and double chain amphiphiles
form micelles and bilayers, respectively. To gain a greater insight into the these processes, we need to understand the thermodynamics
of micelle and bilayer formation. First we must understand the concept of chemical potential, which is related to the free
energy of a reaction.
DG, the free energy chance for a reaction, determines the spontaneity and extent
of a chemical or physical reaction. The free energy of system depends on 3 variables, T, P, and n, the number of moles of
each substance. If the system is composed of two different parts, A and B, the system is at equilibrium (DG=0) if Ta = Tb, Pa = Pb, and dG/dn for A (ma) equals
mb. m is
the chemical potential of a substance, where ma is given
as m=mo +RTln[A].
The chemical potential is really the free energy per mole.
Expressed in more formal mathematical nomenclature, since the natural variables of G and m are T, P, and n, we can write:
dG = (¶G/¶T)P,n + (¶G/¶P)T,n + (¶G/¶n)P,T
where (¶G/¶x)y,z is the derivative (partial) of G with respect to x at constant y and z. That
is the total dG consist of contributions to the change of G from changes in T, P and n.
Since G and m are quite similar, the following series
of analogous equations apply:
DG = DGo
+ RTln Qrx is analagous to Dm = D
mo + RTlnQrx
where Qrx is the reaction quotient = [P][Q]/[A][B] for the reaction A+B<==> P+Q
D G = D H -
TDS is analogous to Dm =
DH - TDS
DGo = DHo
- T DSo is analogous to Dmo = DHo - T D So
DGo = - RTlnKeq is analogous to Dmo = - RTln Keq
Thermodynamics of Micelle and Bilayer Formation
First, let's consider the thermodynamics of micelle formation, based on the equation below:
n SCA <==> 1 micelle
where SCA represents a single chain amphiphile. At first glance we might suspect that:
- DHo < 0 since
the van der Waals interactions among the buried acyl chains in the micelle would be much more favorable than the water - acyl
chain interactions for the monomeric amphiphile in solution. This notion is supported by our aphorism, "like dissolves
like". Of course we couldn't ignore polar interactions (H bonding for example) among the head groups and water, but
we might expect these to be equally favorable in both the monomeric and micellular states.
- DSo < 0 since we are forming a
state (a single micelle) with much less positional entropy from a state (single chains amphiphiles dispersed in solution)
with much more positional entropy. .
Hence it would appear that micelle formation is enthalpically favored but entropically disfavored.
Lets examine this issue more closely. First we need to obtain a greater understanding of Dmo which should give us a clue as to where a SCA would "want" to be in this
mixture. Remember, Dmo depends
only on the relative stability of a molecule in a given environment and not its concentration.
Traube, in 1891, noticed that single chain amphiphiles tend to migrate to the surface of water and decrease
its surface tension (ST.) He observed that the decrease in ST is directly proportional to the amount of amphiphile, added
up until a certain point, at which added amphiphile has no additional effect. In other words, the response of ST saturates
at some point.
We are more interested in what happens to amphiphiles in bulk water, not at the surface. As we mentioned
earlier, monomeric single chain amphiphile is in equilibrium with single chain amphiphiles on micelles. Assume you have
a way to measure monomeric single chain amphiphile in solution. What happens to its concentration as you add more and
more SCA to the mixture? Turns out you observe the same effect that Traube noted with changes in surface tension. This
explanation goes like this: as more amphiphile is added, more goes into bulk solution as monomers. At some
point, there are enough amphiphiles added to form micelles. After this point, added amphiphiles forms more micelles and no
further increases in monomeric single chain amphiphile are noted.. The concentration of amphiphile at which this occurs is
the critical micelle concentration (CMC).
Figure: Graph showings distribution of single chain amphiphiles in bulk aqueous
solution
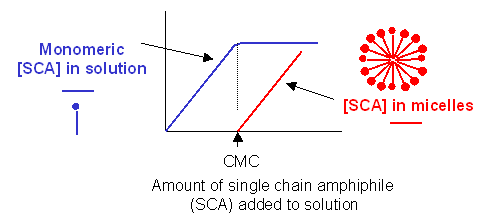
This saturation effect can be observed with other systems as well.
- measure the amount of NaCl(aq) in solution as more NaCl(s) is added to water. At some point, the water
is saturated with dissolved NaCl, and no further increase in NaCl (aq) occurs.
- measure the amount of a sparing soluble hydrocarbon (HC) into water. After saturation, phase separation
occurs.
Consider the example of adding a great excess of NaCl(s) to water. At the very time the salt is added,
the system is clearly not in equilibrium. Therefore, D m < 0. Write the
reaction as follows:
NaCl(s) ------------> NaCl (aq). Let x stand for NaCl. Therefore,
Dm = m x(aq)
- m x(s) = mo
x(aq) + RT ln x(aq) - (mo x(s) + RT ln x(s))
In this equation, all terms are constant except RT ln x(aq), since all the D
mo values are constant, independent of concentration. Remember,
RTln x(s) is constant since the concentration of a solid is constant. With time, Dm becomes less negative, until the system reaches equilibrium, and Dm
= 0. The term RT ln x(aq) becomes more positive since the x(aq) is increasing. When m x(aq) = m x(s) , no further NaCl dissolves.
Remember, a condition for equilibrium is ma = mb.
Consider another example. Add a hydrocarbon x, to a biphasic system of water and octanol. At t = 0, the
system is not at equilibrium. A simple favorable reaction can be written for this system x aq -------> x oct.
Clearly, Dm < 0. Also, Dmo
< 0, since this term is independent of concentration and depends only on the intrinsic stability of x in water in comparison
to that of octanol. The following equation holds as well:
Dm = m x(oct)
- m x(aq) = mo
x(oct) + RT ln x(oct) - (mo x(aq) + RT ln
x(aq)) =
Dm = (mo
x(oct) - mo x(aq) ) + RT ln (x(oct)/x(aq))
=
Dm = Dmo
+ RT ln (x(oct)/x(aq)) .
Figure: Dm for transfer of a sparingly soluble
liquid solute into water
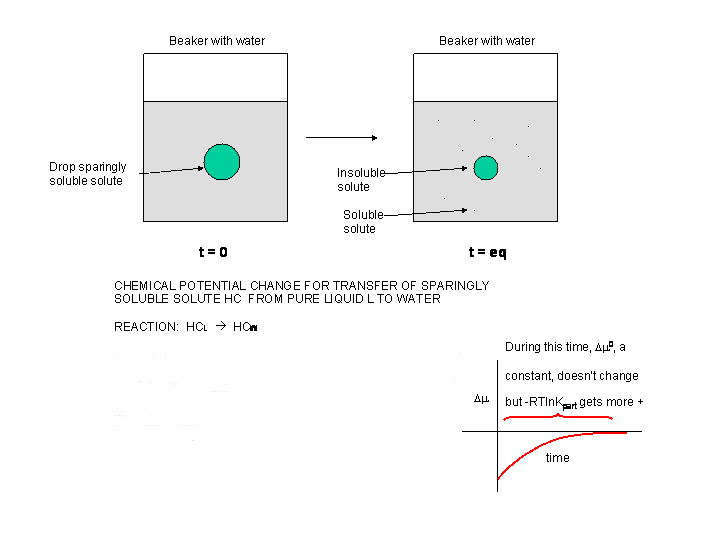
Most of the time, we will be interested in Dmo ,
which depends only on the relative stability, in this case, of x in octanol vs water. Clearly, in this case, Dmo < 0. At equilibrium Dm =
0. By setting the last equation equal to 0, we can see that
Dmo = - RT ln (x(oct)/x(aq))
where the concentration are equilibrium concentrations. We can rewrite this equation as
Dmo = - RT ln Keq
where Keq is the equilibrium partition coefficient for X in octanol and water. This can readily
be determined in the lab. Just shake a separatory flask with a biphasic system of octanol and water after injecting a bit
of X. Then separate the layers and determine the concentration of x in each phase. Plug these numbers into the last equation.
Remember, you should be able to predict the sign and relative magnitude of D mo since it does not depend on concentration, but only on the intrinsic stability of the molecules in
the different environments.
 WebCT Online Quiz (Password required): Driving Forces
WebCT Online Quiz (Password required): Driving Forces
Now we can apply our understanding of Dmo
to the formation of micelles and bilayers. Remember,
Dmo
= D Ho - T D So.
The diagram below shows the standard free energies of transfer of a hydrocarbon X from aqueous solution
to a pure liquid hydrocarbon (HC), x (aq) --------> x (HC).
Dmo = mo
x(HC) - mo x(aq) , which should
be less than 0. In each graph, Dmo is less than 0, and the value
of Dmo decreases (gets more negative) in a linear fashion with
increasing numbers of C in the alkyl chain. Notice the lines are unbelievably straight and parallel. Nature is speaking
is us in these figures. By figuring out the surface area of the chains and the decrease in Dmo with each added CH2 (methylene group), one can calculate that the D
mo decreases by 25 cal/sq. angstrom of hydrocarbon chain added.
Figure: Standard free energy of transfer of HC from aqeuous solution to a pure l
iquid hydrocarbon.
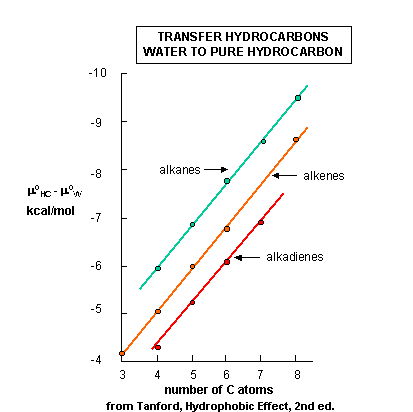
We expected that Dmo for the transfer of x
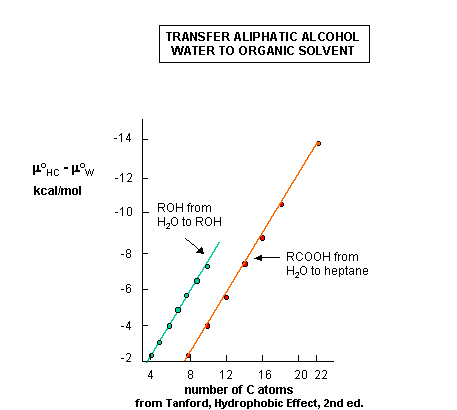
to a pure liquid HC would be negative. The same is true for the transfer of aliphatic alcohols and fatty acids, both
single chain amphiphiles, to organic solvents, as shown in the figure linked below. We could get more information if we would
determine the entropic and enthalpic contributions. Such data is presented in the table below, which shows the
transfer of single chain alcohols from the pure liquid to water
(the opposite of the previous figures.) We expect the Dmo to be
greater than 0 for these cases, since it should be disfavored. What is perplexing about this data is not that it is disfavored,
but that the process is enthalpically favored . This seems to be counterintuitive since it goes against the adage that
like dissolves like as was surmised earlier. From an enthalpic point of view, the amphiphiles prefer (albeit marginally)
to be in water. What makes this reaction disfavored is entropy. The data shows that the aliphatic chains would prefer
not be in water because it is disfavored entropically. This seems to go against our notion from above that when single chain
amphiphiles form micelles, it should be entropically disfavored. This chart shows us the if we were to take a single chain
amphiphile from water into a micelle, (again the opposite of the direction used in this chart), that the entropy would be
positive, and hence be favored!
Figure: Transfer of Aliphatic Alcohols and Fatty Acids from Water to Pure Liquid;
Thermodynamic Parameters for Transfer of Aliphatic Alcohols From the Pure Liquid to Water.
The table below shows the thermodynamic parameters for transfer of HC from water into the interior of
sodium dodecyl sulfate (SDS) micelles and from water to a pure HC liquid. Both of these processes are favored, since D m is less than 0. Notice once again what favors the process of a HC transferring from
water into a micelle (which models at least part of micelle formation) is entropy not enthalpy. Enthalpy disfavors this process
or, at minimum, is neutral.
How can we explain this counter-intuitive favorable entropic process. Again, at first glance it seems
that entropy should disfavor micelle formation, since a single multimolecular aggregate of lower positional entropy (fewer
possible microstates for amphiphile dispersal) is formed from many SCA. However, this is only part of the process. What we
haven't considered is the entropy of water. To place a HC in water, a literal cavity in the water must be created that will
accommodate the HC. Creation of this more ordered cavity must be entropically disfavored (again because the process proceeds
to a state with fewer microstates and lower positional entropy) . Now transferring the HC from water to a micelle will dissipate
the cavity, and lead to more available microstates for the released solvent, bulk water. It is this entropic contribution
that favors micelle and hence bilayer formation. Image this scenario. When you place a HC group in water, water still wants
to maintain its hydrogen bonding. Hence it is forced into a more ordered structure around the HC to maintain its H-bonding,
characterized by fewer microstates. This effect, in which the spontaneous formation of micelles and bilayers is favored by
the increase in entropy when water structured around the nonpolar part of the lipid is freed as the nonpolar part sequesters
into the micelle and bilayer is call the Hydrophobic Effect.
Thermodynamic Parameters for Transfer of Aliphatic Alcohols from the Pure Liquid
to Water at 25oC. (enthalpy determined by calorimeter)
| alcohol |
m w0-m ROH0
(cal/mol) |
H w0-H ROH0 (cal/mol) |
S w0-S ROH0 (cal/deg mol) |
(Cp)w0-(Cp)ROH0 (cal/degmol) |
| ethanol |
760 |
-2430 |
-10.7 |
39 |
| n-propanol |
1580 |
-2420 |
-13.4 |
56 |
| n-butanol |
2400 |
-2250 |
-15.6 |
72 |
| n-pentanol |
3222 |
-1870 |
-17.1 |
84 |
Thermodynamic Parameters for Transfer of Hydrocarbons from Water to Interior of Dodecyl Sulfate Micells
and from Water to Pure Liquid Hydrocarbon State
| Hydrocarbon |
Water --> Micelle Interior |
Water --> Pure Liquid Hydrocarbon |
| DGo (kcal/mol) |
DHo (kcal/mol) |
DSo (cal/deg mol) |
DGo (kcal/mol) |
DHo (kcal/mol) |
DSo (cal/deg mol) |
| Ethane |
-3.45 |
+2.0 |
+18.3 |
-3.9 |
+2.5 |
+21 |
| Propane |
-4.23 |
+1.0 |
+17.5 |
-4.9 |
+1.7 |
+22 |
| Butane |
-5.13 |
0 |
+17.2 |
-5.9 |
+0.8 |
+23 |
from Tanford, The Hydrophobic Effect (New York: Wiley, 1973)
How can we explain the favorable enthalpic contribution to place a nonpolar molecule into water? Again,
this goes against our adage of "like dissolves like". The negative DH suggests interactions
among all the participants are more favorable when the nonpolar group is in water. One source of such interactions could
be the highly structured water in the "cage" that surrounds the nonpolar molecule. If it were more structured than bulk
water, hence more "ice-like" in nature, then the formation of these extra H-bonds would contribute to the negative enthalpy
change. When the nonpolar molecule is removed from the water, a process which proceeds with a positive DH,
the cage of "ice-like" water would "melt", which, like the melting of ice, is not favored enthalpically. Heat energy must
be supplied to break the H-bonds as ice changes state to liquid water.

