|
INTRODUCTION
What types of intermolecular forces might act within a protein and between proteins and solvent molecules
that would cause a protein to fold spontaneously to a unique 3D structure? These forces can be long range (ion-ion, ion dipole,
or dipole-dipole) or short range (van der Waals repulsive and attractive forces). The interactions can be local (between adjacent
amino acids in the linear sequence) or nonlocal (between sequences separated in the linear sequence but brought close together
in 3D space). Clues as to what stabilizes the tertiary structure of a native protein can be gained by subjecting proteins
to agents that unfold or denature a protein. Such agents include extremes of pH, high concentrations of some salt solutions
or organic solvents, and temperature extremes. Such experiments show that native proteins are only marginally stable (about
0.4 kJ/mol amino acid - or around - 10 kcal/mol for a protein of molecular weight of 10,000 - about 100 amino acids). We will
consider the different types of intermolecular forces (ion-ion, H bonds, van der Waals, and the hydrophobic effect) individually
and ask the question: Is this particular IMF the main driving force for protein folding?
Figure: Diagram showing relative contributions to the DG for protein folding.
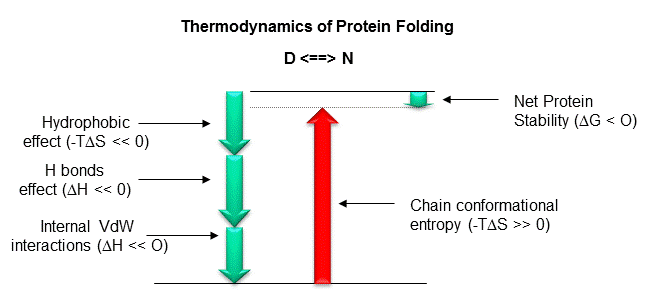
1. Ion - Ion (Electrostatic):
These could be investigated by altering pH or ionic strength. Why is that?
a. General Charge Interactions - Proteins denature at extremes of pH. At these extremes, proteins
have a maximal positive or negative charge, as evident in graphs which show denaturation temperature vs pH for proteins.
Figure: Denaturation temperature vs pH for proteins
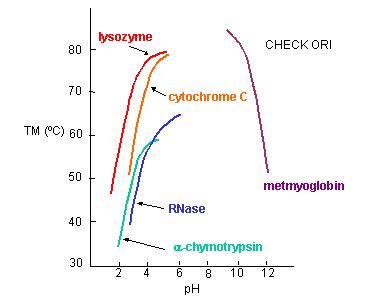
Electrostatic repulsions would cause the protein to denature. The folded, compact state has an increasing
charge density at pH extremes, which could be alleviated by unfolding to a less dense state. But what about specific charge
pair interactions? In contrast to the general charge interactions, these might actually stabilize a protein. Are they the
predominant factor that determines stability?
b. Specific Charge Interactions (charge pairs) - If ion pairs are the source of protein stability,
you would expect that high salt could disrupt them, and lead to denaturation. Although some salts do denature proteins, other
stabilize them. Other evidence argues against this idea. Ion pairs are not conserved in evolution. In addition, the number
of ion pairs in proteins is small (approx. 5/150 residues, with one of those buried). Also, the stability of a protein shows
little dependence on pH or salt concentration (at low concentrations) near the isoelectric point, the pH at which proteins
have a net zero charge.
The next two sections deal with H bonding and the hydrophobic effect. A theme of the course is that if you can understand
the interactions among small molecules, you can apply that knowledge to the understanding of larger molecules like proteins.
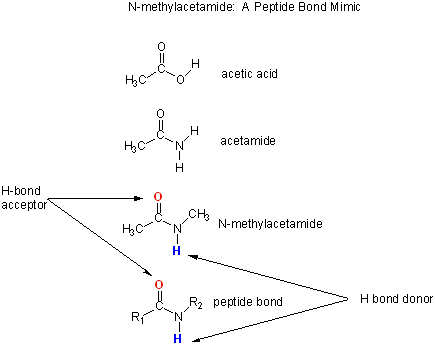
To understand if H bonds within proteins, often buried in the more hydrophobic interior of the protein, drive protein folding,
we will examine the thermodynamics of H bond formation of a small molecule, N-methylacetamide, in water and in a nonpolar
solvent. To understand if the hydrophobic effect, mediated by burying of nonpolar side chains within the more nonpolar
center of the protein, drives protein folding, we will examine the thermodynamics of benzene solubility in water.
2. Hydrogen Bonding
Linus Pauling first suggested that H bonds (between water and the protein and within the protein itself)
would play a dominant role in protein folding and stability. It would seem to make sense since amino acids are dipolar and
secondary structure is common. Remember, however, the H bonds would be found not only in the native state but also in the
denatured state. Do they contribute differently to the stability of the D vs N states? Many experimental
and theoretical studies have been performed investigating helix <===> (random) coil transitions in small peptides. Remember
all the intrachain H bonds in the helix? Are they collectively more stable than H bonds between water and the peptide
in a (random) coil?
Early models assumed that intrachain H bonds were energetically (enthalpically) more favorable than H
bonds between peptide and water. But to form an H bond requires an entropy payback since a helix is much more ordered (lower
entropy) than a random coil (higher entropy). At low temperature, enthalpy predominates and helix formation in solution is
favored. At high temperature, the helix is disfavored entropically. Imagine the increased vibrational and rotational states
permitted to the atoms at higher temperatures. (Remember the trans to gauche conformational changes in the acyl chains of
double chain amphiphiles as the temperature increased, leading to a transition from a gel to liquid crystalline phase in bilayer
vesicles.) Theoretical studies on helix-coil transitions predict the following:
- as the chain length increases, the helix gets more stable;
- increasing the charge on the molecule destabilizes the helix, since the coil, compared to the more compact
helix, has a lower charge density;
- solvents that protonate the carbonyl oxygen (like formic acid) destabilizes the helix; and
- solvents that form strong H bonds compete with the peptide and destabilize the helix. In contrast, solvents
such as CHCl3, dimethylformamide (a nonprotic solvent), or 2-chloroethanol, and trifluoroethanol, which form none
or weaker H bonds to the peptide than does water, stabilizes the helix.
These helix-coil studies suggest that H bonds are important in stabilizing a protein.
But do they really? Why should these H bonds differ from those in water? It's difficult to
figure out whether they are since there are so many possible H bonds (between protein and water, water and water, and protein
and protein), and their strength depends on their orientation and the dielectric constant of the medium in which they are
located.
If intrachain H bonds in a protein are not that much different in energy than intermolecular H bonds between
the protein and water, and given that proteins are marginally stable at physiological temperatures, then it follows that the
folded state must contain about as many intramolecular hydrogen bonds within the protein as possible intermolecular H bonds
between the protein and water, otherwise the protein would unfold.
To resolve this issue, and determine the relative strength of H bonds between the varying possible donors
and acceptors, many studies have been conducted to compare the energy of H bonds between small molecules in water with the
energy of H bonds between the same small molecules but in a nonpolar solvent. The rationale goes like this. If the interior
of a protein is more nonpolar than water (lower dielectric constant than water), then intrastrand H bonds in a protein might
be modeled by looking at the H bonds between small molecules in nonpolar solvents and asking the question, is the free energy
change for the following process < 0:
Dw + Aw <=======> (DA)n, DGo,
K
where D is a hydrogen bond donor (like NH) and A is a hydrogen bond acceptor, (like C=O), w is water (i.e.
donor and acceptor are in water), and n is a nonpolar solvent, and DGo
and K are the standard free energy change and the equilibrium constant, respectively, for the formation of a H-bond
in a nonpolar solvent from a donor and acceptor in water. This reaction simulates H-bond contributions to protein folding,
where a buried H-bond is mimicked by a H-bond in a nonpolar solvent. The reaction written above is really a thought experiment,
since it would be hard to set up the necessary conditions to make the measurement. However, we can calculate the DGo for this reaction since it is a state function and it really doesn't matter
how one accomplishes this process.
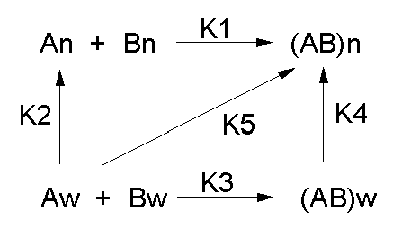
Let's consider a specific example: the formation of H bonds between two molecules of N-methylacetamide
(NMA) in water and in a nonpolar solvent. The reaction scheme shown below describes a set of reactions (a thermodynamic
cycle) involving the formation of H-bonded dimers of NMA . A and B are both molecules of NMA, in either water
(w) or a nonpolar solvent (n).
N-methylacetamide is a good mimic for the H bond donors and acceptors of the peptide bond of a polypeptide
chain.
In the reaction scheme shown above,
K1 is the equilibrium constant for the dimerization of NMA in a nonpolar medium. This can be
readily determined, and is >1, implying that DGo < 0. (Remember,
DGo = -RTlnKeq) For the dimerization of NMA in CCl4,
DGo1 = -2.4 kcal/mol.
K2 is the equilibrium constant (think of it as a partition coefficient) for the transfer of
two NMA molecules from water to a nonpolar solvent (again easily measurable). For NMA transferring from water to CCl4,
DGo2 = + 6.12 kcal/mol.
K3 is the equilibrium constant for the dimerization of NMA in water. This can be readily determined,
and is <1, implying that DGo > 0. For the dimerization of
NMA in water, DGo3 = +3.1 kcal/mol.
K4 is the equilibrium constant (think of it as a partition coefficient) for the transfer of
a hydrogen-bonded dimer of NMA from water to a nonpolar solvent. You try to think of a way to measure that! I can't. This
is where thermodynamic cycles comes in so nicely. You don't have to measure it. You can calculate it from K1-3
since DGo is a state function!
DGo2 + DGo1 = DGo3
+ DGo4 OR -RTlnK2
+ -RTlnK1 = -RTlnK3 + -RTlnK4
lnK2 + lnK1 = lnK3 + lnK4
= ln(K2K1 )= ln(K3K4) or (K2K1 )= (K3K4)
For NMA transferring from water to CCl4, D Go4
= + 0.62 kcal/mol.
(Note: Biochemists like to talk about thermodynamic cycles which may seem new to you. However,
believe it or not, you have seen them before - in General Chemistry - in the form of Hess's Law!)
From K1-4and the corresponding DGo
values, we can now calculate DGo5 for the formation
of H-bonded NMA dimers in a nonpolar solvent from two molecules of NMA(aq). This reaction, which we hope simulates
formation of buried intrachain H bonds in proteins on protein folding, is:,
Dw + Aw <=======> (DA)n, for which
DGo5= +3.72 (i.e. disfavored).
If this model is a good mimic for studying H bond formation on protein folding, it suggests that the formation
of buried H bonds during protein folding does not drive protein folding.
However, if the transfer of D and A (from a large protein)
from water to the nonpolar medium (modeled by K2) is driven by other forces (such as the hydrophobic effect), the
positive value of K1 will strongly favored buried H bond formation. So, if this happens in proteins, it is clear
why so many intrachain H bonds occur, since K1 is so favored. H bonds may not assist the collapse of a protein,
but would favor internal organization within a compact protein. That is, H bonds don't drive protein folding per se,
but form so that the folded protein would not be destabilized by too many unsatisfied H bonds.
There are potential problems with this simple model. The interior of a protein is not homogeneous (i.e.
the effective dielectric within the protein will vary). H bond strength is also very sensitive to geometry. Also, there are
many H bonds within a protein, so slight errors in the estimation of H bond strength would lead to large errors in determination
of protein stability.
Another argument against H bonds being the determining factor in protein folding and stability comes from
solvent denaturation studies. If intrachain H bonds are so important, then should not solvents that can H bond to the backbone
denature the protein? Shouldn't water (55 M) act as a denaturant? It doesn't, however. Dioxane (5 member heterocyclic ring
with O) which has only a H bond acceptor wouldn't be expected to denature proteins, but it does. H bonds also increase in
nonpolar solvents. Peptides which have random structures in water can be induced to form helices when placed in alcohol solutions,
which are more nonpolar than water, as explained above in the helix-coil studies. If H bonds are the dominate factor in protein
stability, the alcohols would stabilize proteins. At low concentrations of alcohol, proteins are destabilized.
Hence, it is unlikely that H bonds are the big stabilizers of protein structure. Only 11% of all C=O's
and 12% of all NH's in protein have no H bonds (determined by analysis of X ray crystallographic structures). Of all H bonds
to C=O, 43% are to water, 11% to side chains, and 46% to main chain NH's. Of all H bonds to NH, 21% are to water, 11% to side
chains, and 68% to the main chain C=O.
3. Hydrophobic Interactions:
We have studied the role of the hydrophobic effect (involving the favorable entropic release of caged water molecules about solvent-exposed hydrophobic groups) in driving micelle
and bilayer formation. Does this also drive protein folding? To explore this questions, we will study the thermodynamics
of small nonpolar molecules, especially benzene, with water and ask whether the thermodynamic parameter associated with
benzene solubility are similar to those associated with protein stability. If this analogy holds, anything that will
promote benzene solubility will lead to increased hydrophobic amino acid side chain exposure to water and hence protein denaturation.
What is the evidence to support this?
a. crystal structures show that most nonpolar side chains are buried inside a protein, which is
tightly packed and which excludes water. Studies show that as the surface area of amino acid side chains increase,
the free energy of transfer of amino acids from water to ethanol becomes more negative.
Figure: Transfer of amino acids from water
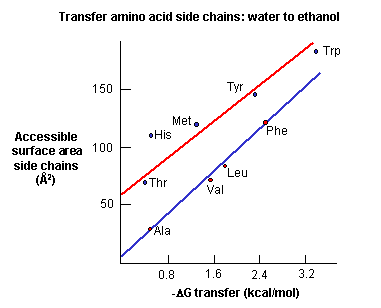
(Review free energies of transfer of hydrophobic groups in Chapter 1E: Lipids in Water - Thermodynamics )
b. low temperature denaturation of proteins - It has been observed that proteins can denature at
low temperatures (less than 0oC), suggesting that nonpolar residues become more "soluble" in water at low temperatures
(i.e. they move from the more hydrophobic interior of a protein to the more polar outside). Compare the solubility of nonpolar
gases like CO2 or N2, which are more soluble at low temperature. As you heat solutions of nonpolar gases
in water, the gases become less soluble as evidenced by bubble formation (i.e. phase separation of dissolved gases as they
become more insoluble). If protein behavior is governed by this same behavior (greater solubility of nonpolar groups at low
temperatures), it would suggest that proteins might denature at low temperatures (leading to increased exposure to water of
the nonpolar side chains). This phenomena has been observed.
c. protein stability affected by different salt species - Over 100 years ago, Hofmeister determined
the effectiveness of different cations and anions of salts to precipitate blood serum proteins in the 0.01 - 1 M concentration
ranges. The series is shown below:
Cations: NH4+ > K+ > Na+ > Li+ > Mg2+
> Ca2+ > guanidinium
Anions: SO42- > HPO42- > acetate > citrate > Cl-
> NO3- > ClO3- > I- > ClO4- >
SCN-
- A salt from pairs of the first ions in these series (for example, (NH4)2SO4),
when added to aqueous solutions of proteins, precipitate the native form of the protein. We must account for
the fact that it precipitates the protein, and that the protein is precipitated in the native, not denatured state.
More on why it precipitates proteins in a moment. The first ion in each series increases the surface tension of water
(making it harder to make a cavity in the water to fit the nonpolar molecule). This decreases the solubility of nonpolar
molecules. These "salt-out" nonpolar molecules, promoting not dissolution in water but aggregation followed
by a phase separation. By analogy, they will stabilize the native state since the buried hydrophobic side chains
would have a decreased propensity to move out into the aqueous environment.
- The last ions of the series have less affect on surface tension, and hence increase the solubility of
nonpolar molecules ("salt-in"). By analogy, they will destabilize the native state since the buried hydrophobic side chains
would have an increased propensity to move out into the aqueous environment.
Figure: Hofmeister Series
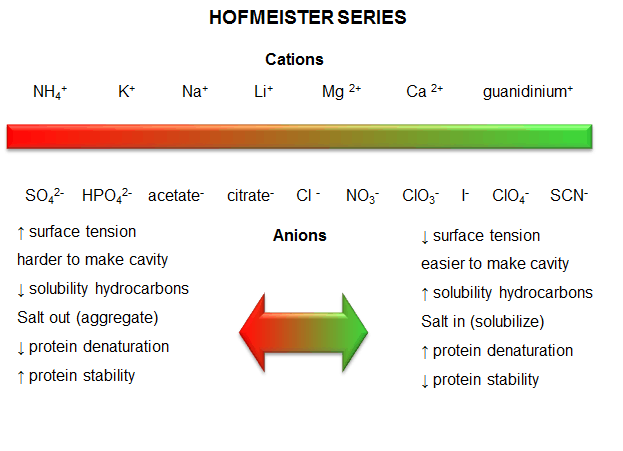
The solubility of benzene in aqueous salt solutions of this series increases from left to right, just
as native protein stability decreases from left to right (i.e. the protein's nonpolar core residues become more "soluble"
in water, leading to its denaturation).
Additives to proteins that increase the stability of the folded state of the protein also tend to decrease
their solubilities. These additives are excluded from the preferential water hydration sphere around the protein (negative
binding of these agents). Denaturants in contrast tend to increase protein solubility and interact preferentially with the
protein surface. In their presence, proteins respond by increasing their surface area by denaturation. For stabilizers, proteins
try to minimize their surface area by staying "native" and aggregating to form a precipitate, both of which minimizes the
surface area from which the stabilizing agent is excluded.
The main effect of dissolved ions on water structure has been thought to involve changes in H bonds (either
enhancers/structure maker or inhibitors/structure breakers) which correlate with salting-in or salting-out effects of various
ions. Many techniques have been used to study these interactions:
- viscosity: inferential information on structure
- diffraction (x-rays/neutrons): gives information on coordination number of solvation shell (static information)
- NMR: information on average relaxation of bulk and hydration sphere water around ions (dynamic information)
- molecular dynamics simulations: which gives insight into short but not long range interactions between ions and water.
Recent studies have provided conflicting support for the notion of structure makers/breakers. New
research (Omta et al. 2003) has used femtosecond mid-infared pump-probe spectroscopy to study actual H-bonds between water
molecules in salt solutions (Mg(ClO4)2, NaClO4, and Na2SO4).
In pump-probe spectroscopy, a sample is excited with a short pulse (pump) and after a short time lag, with another pulse (probe),
which interacts with the excited state. The linear-polarized infrared pulses (pump) were used to excite OH groups in
solution, followed by a probe pulse which was polarized 45 degrees compared to the pump pulse. Only those excited OH
groups that had rotated in the time interval between the pump and probe would be excited by the probe. Using this technique,
the time frame for reorientation of the OH groups, which is related to the "stiffness" of the H bonds, can be determined.
The salts had no effect on the rotation motion of bulk water outside of the the first hydration shell, which suggests that
salts have no effects on the H bond networks in bulk water. Mg2+ ions are considered structure making, as
the ions greatly increases the viscosity of water, brought about supposedly by increased H bonds among water molecules.
This study does not support this model. Increased viscosity of Mg solutions must be attributed to those ions directly
interacting with water molecules. The solution can be modeled as bulk water with small rigid spheres of ion + first
hydration sphere. Clearly, much more experimental and theoretical work must be performed to gain structure insight
into the role of salts on water structure. Until then, we will continue to try to understand the effects of different
salt on water structure in descriptive term and with use of thermodynamic quantities.
d. conservation of hydrophobic core residues - These residues are highly conserved and correlated
with structure.
e. Urea denatures proteins - 8M urea is often used to denature proteins. People used to think that
urea competed with the intrachain H bonds and hence unraveled the protein. The arguments above with H bonds disputes this
contention since water should then denature protein. How does urea denature proteins? It has been shown that the free
energy of transfer of the nonpolar amino acids into 8M urea is increasing negative as the side chains become bigger and more
nonpolar.
Figure: Free energy of transfer of the nonpolar amino acids into 8 M urea
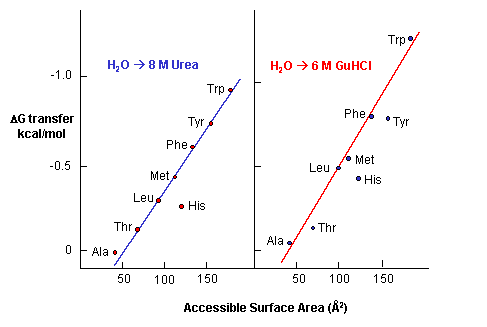
This is also true for denaturation by guanidine hydrochloride. Urea also increases the solubility
of nonpolar molecules in a manner proportional to their surface area.
Apparently urea binds preferentially to the protein surface, and hence tends to increase the protein's
surface area and hydrophobic exposure, and denature proteins. In contrast, ammonium sulfate has the opposite effect.
Figure: How reagents might interact with the surface of the protein.
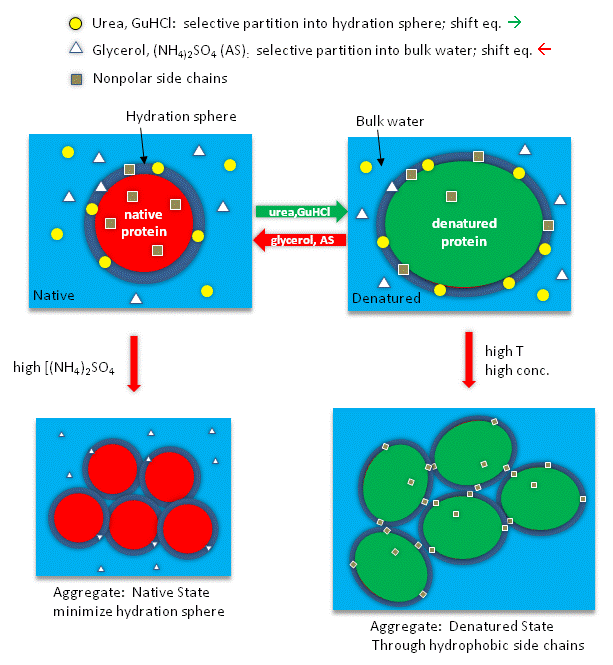
Thermodynamic cycles can make it easier to visualize these transitions, by breaking the denaturation and
perturbant (urea, etc.) interaction into two separate steps, which when added lead to the final state. The next figures shows
such a thermodynamic cycle for urea denaturation of proteins
Figure: Thermodynamic cycle for urea denaturation of proteins
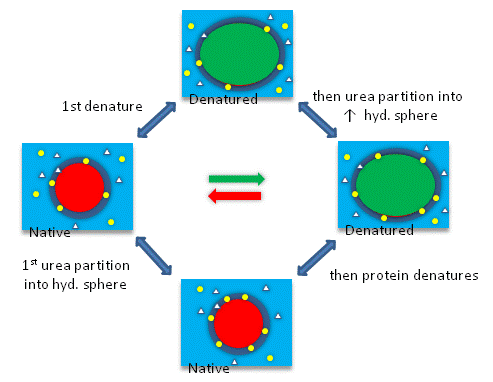
Throughout the semester we will be discussing equilibria and how they may be shifted. The diagram below
shows the first of many diagrams which will show cumulative examples of shifting equilibria.
SHIFTING EQUILIBRIA - 1
Our understanding of hydrophobic interactions has changed dramatically in the last several
years. This is not reflected in most textbooks. The hydrophobic effect mean different things to different people. Some refer
to the transfer of nonpolar solvents to aqueous solution. Some refer to the same phenomena only if the effects have a unique
temperature dependency. Other refer to the ordering of water around nonpolar residues. The most recent explanation centers
around the unique temperature dependencies of the transfer reactions. Before we can understand it, here is an interesting
bit of data. If you dissolve one mole of methane in hexane, the volume of 1 L of hexane changes 60 ml, but if done in water,
the water volume changes 37 ml, indicating that water molecules pack more efficiently around nonpolar molecules then in its
absence.
Let's now consider the thermodynamic aspects of the hydrophobic effect, as we did for micelle
and bilayer formation. In a brief summary, we found the the free energy of transfer of an amphiphile from aqueous solution
into a micelle, for example, was disfavored enthalpically (unexpectedly) but favored entropically (also unexpectedly until
we included solvent in our model). These experiments were done at one temperature and gave us our first initial understanding
of the hydrophobic effect. We will expand on this view by looking at the enthalpic and entropic contribution to the
transfer of benzene into water as a function of temperature. This will lead us to a more modern view of the hydrophobic
effect.
THERMODYNAMICS OF MIXING TWO SUBSTANCES
If you mix two substances A and B that aren't very soluble in each other, two opposing forces are relevant.
- The tendency to mix is driven by an increase in entropy.
- The mixing is usually opposed by enthalpy.
The later makes "intuitive" sense since you might expect that van der Waal forces between A-B might be
less than those of A-A and B-B (i.e. the old adage "like dissolves like"). If AA and BB self interactions are stronger, A
would not dissolve in B and vice/versa. You would also expect no significant changes in entropy and enthalpy as a function
of temperature in this ideal mixing. See the graph below.
The most modern understanding of the hydrophobic interactions shows that we have mixing of A and B, but
with a unique temperature dependency for the value of the change in entropy and enthalpies. At room temperature, if one corrects
the entropy changes for effects due just to mixing, the "excess" entropy is what principally opposes taking a nonpolar molecule
into water. Enthalpy changes are small. We have modeled this effecting using structured water around the nonpolar residues.
Remember our discussions of micelle and liposome formation? We will further our understanding of the hydrophobic effect
by studying benzene solubility in water.
TRANSFER OF BENZENE TO WATER AS A FUNCTION OF TEMPERATURE: A MODEL SYSTEM
Before we discus entropy and enthalpy changes accompanying protein folding/unfolding, let's try to learn
about the thermodynamic aspects governing benzene solubility in water. What happens to benzene solubility in water and
the corresponding thermodynamic parameters as you raise the temperature? The graph below shows the change in G, H and -TS
for taking benzene from pure benzene to water. This is real data.
Figure: change in G, H and -TS for taking benzene from pure benzene to water.
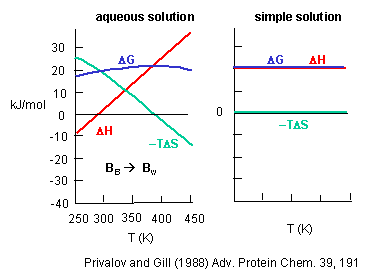
Notice the temperature dependency of these thermodynamic variables. As temperature increases, -TDS becomes more negative and hence drives benzene into water. (Remember D
G = D H - TDS.) This seems to contradict our understanding of entropy effects in micelle and bilayer formation
that we learned earlier when the DS > 0. Those studies on micelle
and bilayer formation were done at one temperature. What about the structuring of water around the nonpolar group
(decreasing the number of available microstates) hindering the process? Rationalize it this way. As the temperature is raised,
available positional and thermal entropy of water increase significantly. . It would seem logical that to then put a
nonpolar residue into this system of water would become easier than putting it into more structure water (characterized by
fewer microstates and lower positional and thermal entropy) at a lower temperature! (Remember from our review
of thermodynamics that If the Tsurr is high, a given heat transfer to or from the surroundings will have a smaller effect on the ΔSsurr; conversely, if
the Tsurr is low, the effect on ΔSsurr will be greater. Atkins, in his recent General Chemistry,
uses the analogy of the effect of a sneeze in library compared to in a crowded street;
The new American Chemistry General Chemistry text uses the analogy of giving $5 to a friend with $1000 compared to one who
has just $10.)
But look at the other temperature anomaly. It becomes increasingly difficult from an enthalpic point of
view to put benzene in water. We saw with micelle experiments that putting a hydrophobe into a micelle was disfavored enthalpically
(and hence favored enthalpically to put it into water). At a high temperature, -TDS becomes zero, and their is no entropic barrier to putting benzene into water. The barrier is completely enthalpic.
This is why a more nuanced definition of the hydrophobic effect has emerged.
If you sum DH and -TDS at each temperature, you get the curve shown for the total DG
to take benzene from pure benzene to water. Notice that it is always positive, so it is always disfavored. The DG function is curved. It increases at low temperatures, and decreases at very high
temperatures, implying that there will be a temperature at which there is a minimum solubility of benzene in water (a maximum
in the positive DG). The minimum solubility of benzene (the max. positive
DG) occurs when dG/dT = 0. Now remember from physical chemistry that
dG = VdP - SdT, so that dG/dT = V dP/dT - S = -S.
Therefore, dG/dT = 0 occurs when S = 0, and the maximum aversion is driven by enthalpy. This is
at variance with the view that water ordering is the principal feature of the aversion of nonpolar residues for water.
Let's review some more thermodynamics that the physical chemists in the crowd should remember. Even General
Chemistry students should to a degree (a pun). Heat capacity, Cp, is defined as the heat required to raise
the temperature of a mole of a substance 1oC. It has units of kJ (or kcal)/o mol. Look at the slope
of the enthalpy curve as a function of temperature. It has units of kJ/o mol or of heat capacity and is given
by:
Cp = dH/dT.
(Earlier, we studied Cp vs T curves for the phases transitions of water and for the gel/liquid crystalline
phase transitions of phospholipid vesicles
Figure: Phase transitions of water
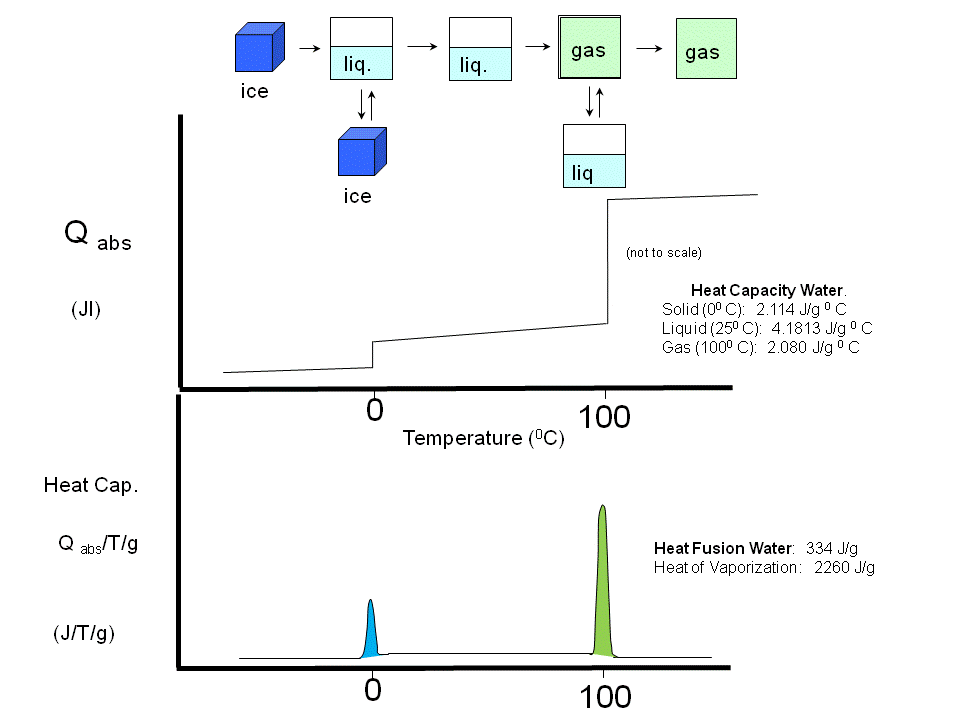
Figure: gel/liquid crystalline phase transitions of phospholipid vesicles
Here is yet another equation for heat capacity that you derived in physical chemistry:
Cp = dH/dT= TdS/dT.
The last equality stems from Maxwell's relationships which Physical Chemistry students should remember.
By analogy,
DCp = d(DH)/dT
= Td(DS)/dT
which is POSITIVE in the benzene graphs above. THIS POSITIVE DCp OBSERVED WHEN A HYDROPHOBIC GROUP IS TRANSFERRED TO WATER
IS THE SIGNATURE FOR OUR NEW DEFINITION OF THE HYDROPHOBIC EFFECT!
A positive DCp occurs when DH and DS are dependent on temperature, which is observed when
a hydrophobe is transfer from a more nonpolar environment to water. Likewise, a negative DCp is observed when hydrophobes in water are transferred to a more nonpolar environment. Look at the graph showing
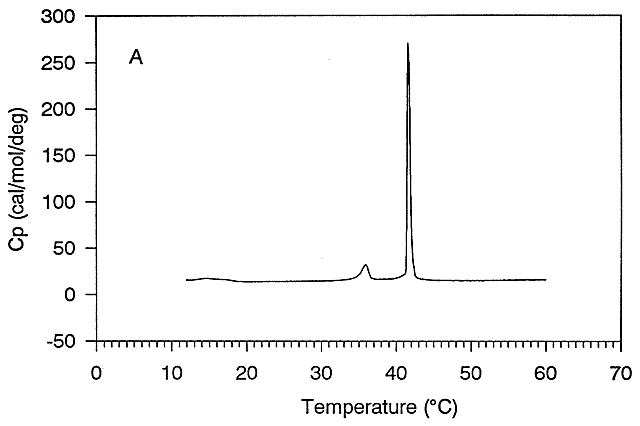
the heat capacity of proteins vs temperature obtained using differential scanning calorimetry, as we previously studied with
lipids.
Figure: heat capacity of proteins vs temperature
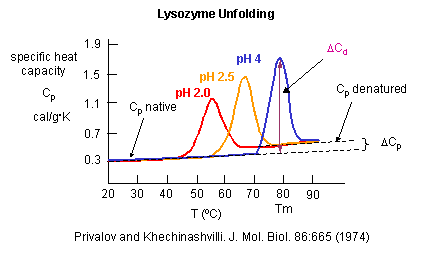
As the protein is heated, it reaches a temperature at which a large amount of heat is suddenly absorbed,
as the protein unfolds. The area under the curve represents the heat absorbed. The temperature at the midpoint is the Tm of
the protein. (Why would the Tm be dependent on the pH of the solution?) Finally the protein denatures. Notice that there are
two DCp's evident. One DCd
is associated with the actually denaturation process. The other is D Cpd
which shows the difference between the heat capacity between the denaturated and native state. Notice that both are positive,
suggesting the transfer of hydrophobes from the interior of the protein to water.
What is the molecular basis for this large heat capacity change of transfer for benzene. One can show
that the Cp is also proportional to the surface area of the nonpolar solute. At room temperature, water molecules surrounding
the nonpolar residue are low in energy (lots of H bonds) and low in entropy (thermal and positional , fewer available microstates).
As the temperature is raised, water populates higher energy states (fewer H bonds) and higher entropy (thermal and positional
, more available microstates. The increase in temperature causes "melting" of surrounding water structure in so far as energy
and entropy are concerned. The two different energetic states of water provide an energy storage mechanism.
Consider a slightly different explanation. Water molecule form an "iceberg"-like cage of water around
nonpolar molecules, which is often called a clathrate. The water is fully H-bonded (to itself, not to the nonpolar molecule)
in a fashion analogous to ice but the geometry of the H bonds is nonideal. This strucuturing of water decreases its entropy.
With increasing temperature, the structured water "melts" which produces the large heat capacity of a solution of a nonpolar
molecule in water, just as the actual melting of ice showed a large heat capacity. This large heat capacity is the signature
thermodynamic features of the solution of a nonpolar molecule in water.
RELATIONSHIP BETWEEN BENZENE TRANSFER TO WATER AND PROTEIN DENATURATION
The graph above shows a maximum in benzene insolubility. As the temperature is decreased from that
maximum, benzene becomes more soluble in water. Alternatively, as temperature rises to that temperature of maximal
insolubility, the solubility of benzene decreases (just like nonpolar gases become increasingly insoluble with increasing
temperature). If you extrapolate the DG curve in this range
of decreasing temperature past the range shown on the graph, it would cross the X axis and become <0, implying benzene
would be favored to dissolve in water. Does the low temperature behavior of benzene/water interactions (becoming
more soluble as the temperature is decreased from the maximum temperature for its insolubility) extend to and predict protein
behavior at low temperature? (The following figures shows the analogy between benzene solubility in water and protein denaturation.
Figure: analogy between benzene solubility in water and protein denaturation
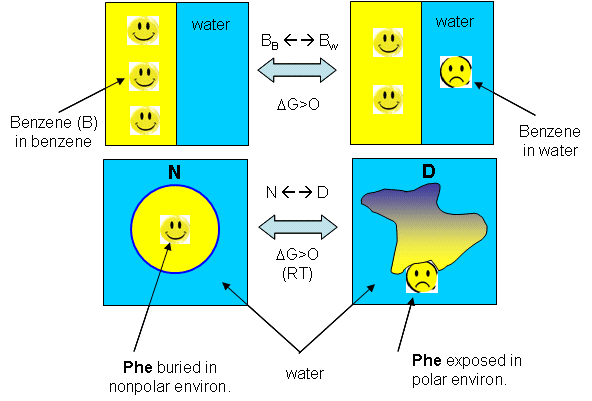
In the figure, F stands for a Phe side chains, which can be buried, sequestered from water as it would
be in the native state of the protein, and exposed to water, as it might be in the denatured state.) The answer is yes, at
low temperature. The analogy to benzene being more soluble at low temperature is the hydrophobic side chains in a protein
becoming more likely to flip into water, denaturing the protein. The low temperature behavior would predict low temperature
protein denaturation. This phenomena has been observed.
What about high temperature? Proteins denature as the temperature increases to the range that the DG curve for benzene reaches a peak. If the hydrophobic residues behave like benzene they
would like to stay buried and not flip out into water as the temperature rises to the maximum in the DG curve. Hence this predicts that the protein should become more stable. What then explains the denaturation
of proteins at high temperature? Another factor must account for it. What is it?
Remember the trans to gauche conformational changes in fatty acid residues in liposomes? As the temperature
is increased, more conformations become available and occupied. Consider a protein. At low temperature, their is only
one native state and to pick a number, maybe 100 accessible denatured states. At high temperature, there is still only one
native state, but possibly 1000 accessible denatured states. More accurately, think of the protein existing in an ensemble
of conformations. As the temperature increases, more denatured states can be populated, compared to at lower temperatures,
leading to an entropic driving force favoring unfolding. Which way would the chain conformational entropy drive
the protein at high temperature? Clearly, it would be driven to the most number of states - to the denatured state.
Hence a modern definition of the hydrophobic effect can explain low temperature denaturation, but not high temperature denaturation.
Remember when we discussed the thermodynamics of transfer of aliphatic alcohols from water to the pure
alcohol? We decided that DGo was < 0 (favorable), and that
DHo > 0 (disfavorable) and DSo > 0 (favorable). Also remember that these figures were derived at one temperature. We were somewhat
surprised that DHo > 0 since this implies that from an enthalpic
point of view, the alcohol-water interactions, or the water-water interactions surrounding the hydrocarbon chain are more
favorable than the alcohol-alcohol interactions or bulk water-water interactions. The freeing of structured water surrounding
the aliphatic chain when the alcohol is transferred to the pure alcohol is the driving force for the reaction. What happens
at different temperatures? I hope it makes intuitive sense that the entropy effects will change with temperature, as described
above. Likewise it makes sense that the enthalpy would change. Hence DH and
DS for the transfer of amphiphiles into water will be a function of temperature
- i.e. the reaction proceeds with a DCp.
Web Links:
 Online Literature: Southall, N., Dill K., and Haymet, D. A. View of the Hydrophobic Effect. J. Phys. Chem. 106, pg 521 (2002) Online Literature: Southall, N., Dill K., and Haymet, D. A. View of the Hydrophobic Effect. J. Phys. Chem. 106, pg 521 (2002)
Summary of studies from small molecules (N-methyacetamide
and benzene)
It is clear that proteins are not all that stable, and many contributions of varying magnitudes must sum
to give the proteins marginal stability under physiological conditions. Hydrophobic interaction, defined
in the new sense, must play a major role in stability. Also, since proteins are so highly packed compared to a lose denatured
state, London Forces must also play a significant part. (Remember dispersion forces are short range
and become most significant under conditions of closest packing.) Opposing folding is the chain conformational entropy
just described. Since proteins are so marginally stable, even one unpaired buried ionic side chain, or 1-2 unpaired buried
H bond donors and acceptors in the protein may be enough to "unravel" the native structure, leading to the denatured
state.
New studies on protein stability
Site Specific Mutagenesis Studies
In the last decade, the contributions to the overall stability of a protein from the hydrophobic effect and H bonds has
been studied using site specific mutagenesis. In this technique, the DNA coding sequence for a given amino acid in a
gene can be altered so that the new mutant protein differs from the normal protein (often called the wild type protein)
by one amino acid. To probe the hydrophobic effect, for example, a buried hydrophobic amino acid like Ile could be changed
to Gly which is much smaller, and offers less hydrophobic contribution to the stability of the native state. The result
of this mutation might leave a "hole" in the protein (not unlike the vacant holes in crystal structures of salts that you
studied in General Chemistry). This "hole" might be diminished in size by subtle rearrangement of the protein structure
in the vicinity. Certain amino acids would not be used as replacements in such studies. For instance, an
Ile would not be replaced with a positively charge Arg which would clearly destabilize the protein. The extent of destabilization
in mutant proteins can be determined by calculating the DGo for the native to denatured transition using urea as the denaturing agent.
Previously, the following statistics were presented concerning the distribution of amino acids in the tertiary structure
of a protein. New values are shown below in red, based on much more crystallographic data, as summarized in Pace's article.
- The side chain location varies with polarity. Nonpolar side chains, such as Val, Leu, Ile, Met,
and Phe, are nearly always (83%) in
the interior of the protein.
- Charged polar side chains are almost invariably on the surface of the protein. (54% - Asp, Glu, His, Arg, Lys are buried away from water, a bit startling!)
- Uncharged polar groups such as Ser, Thr, Asn, Gln, Tyr, and Trp are usually on the surface, but frequently
in the interior. If they are inside, they are almost always H bonded (63%
buried - Asn, Gln, Ser, Thr, Tyr, again startling) .
- Globular proteins are quite compact, with water excluded. The packing density (Vvdw/Vtot)
is about 0.75, which is like the NaCl crystal and equals the closest packing density of 0.74. This compares to organic liquids,
whose density is about 0.6-0.7.
Two articles by Pace suggests that Dills "influential review (from which much of the above derives)
that concluded that hydrophobicity is the dominant force in protein folding" should be rethought. Using site specific
mutagenesis to change Asn (which can H bond through its side chain) to Ala (which can't) in a variety of proteins, he has
shown that approximately 80 cal/mol/A3 of stability is gained if a side chain (in this case Asn) can form buried
H bonds to buried amide links of the protein backbone. Similar studies of mutants in which Leu is replaced with Ala,
and Ile with Val, suggests that only 50 cal/mol/A3 is gained from burying a hydrophobic -CH2- methylene
group. Extending these results to protein folding suggest that proteins stability is determined more by the formation
of buried H bonds than by the hydrophobic effect.
The investigators measured DGo for the N <=> D transition (presumably by varying
the urea concentration and extrapolating the DGo for unfolding to 0 M urea (see: Lab Determination of DGo of Unfolding). For the reaction as written, DGounfolding > 0 at room temperature
and 0 M urea. The mutant protein, since they are destabilized, would have a less positive value for DGounfolding
(They would also have a less negative value for folding since they are less stable). The difference in DGounfolding
between the wild type and mutant (DDG) is expressed as:
DDG = DGounfolding wild-type
- DGounfolding mutant > 0
DDG > 0 since
DGounfolding
wild-type > DGounfolding mutant. The more positive
the DDG, the more the mutant is destabilized in comparison to
the wild type. The data for a series of mutants is shown below.
Analysis of Mutants: H Bonds in Protein Folding
| mutation |
DVol side chain (A3) |
% buried |
DDG (kcal/mol)
(destabilized) |
DDG (cal/mol/A)
(destabilized) |
| Asn to Ala |
37.4 |
95 |
2.9 |
78 |
| Leu to Ala |
74.5 |
99 |
3.6 |
48 |
| Ile to Val |
25.8 |
100 |
1.3 |
50 |
What leads to protein stabilization/destabilization when Asn is changed to Ala? One factor which would actually stabilize
the mutant (Ala) protein is conformational entropy of the residue Since Ala would find itself in a larger "hole"
and have greater freedom for motion, it would have more conformational entropy that would stabilize the mutant over the wild
type. Hence the observed destabilization in the Asn to Ala mutant would be even greater if this were taken into account.
In the proteins he studied, only one of eight Asn to Ala mutation involved an Asn in a helix, so the average change could
not be attributable to differences in helix propensities for the two amino acids. In the mutant, assuming no rearrangement
of the remaining side chains, there is an "unnecessary" and unoccupied 37.4 A3 cavity. To create this cavity
is thermodynamically unfavorable (about 22 cal/mol/A3 obtained from values for hydrophobic mutations). If
the same penalty were applied here, the Asn to Ala mutant would be destabilized by 0.8 kcal/mol (22 x 37.4), (less than observed).
Even if there were compensatory changes to minimize the cavity size, this again would only help to stabilize the protein.
These possible explanations that might lead to stabilization/destabilization of the mutant are summarized in the table below.
Possible Explanation of Destabilization of Asn to Ala Mutants
| possible reasons |
explanation |
effect on mutant |
support observed destab. of mutant? |
| residue conformational entropy |
Ala in bigger hole: more freedom motion, favored entropically |
stabilize mutant |
opposite observed. |
|
free energy excess cavity formation |
energy penalty to make an unoccupied cavity
approx. 0. 8 kcal/mol
|
destabilize |
support observed but not size of observed effect (2.9 kcal/mol |
| free energy change for protein conformational changes |
rearrange protein to fill cavity |
stabilize mutant |
opposite observed |
Hence these alternative sources to explain the destabilization of the mutant can't account for the data and we're left
with the explanation that the stability of the native protein over the mutant is accounted for by burying the H bond donor
and acceptors of the amide group and associated changes in van der Waals interactions.
Pace argues that burying the amide group of Asn is similar to burying the peptide bond of the main chain. There sizes
are very comparable. Free amide groups can form four H bonds, but peptide (amide) groups can only form three.
Even if the value of 78 for the DDG (cal/mol/A) is adjusted for this, the new value of 62 is still larger than that for burying a methylene group.
Analysis of 108 folded proteins has shown that hydrophobic groups contribute 118,200 A3 of buried
volume, compared to 92,000 A3 for peptide groups. Multiplying these figures by 78 and 49 (from the above
table) suggests that overall, burying peptide groups contributes more to protein stability than burying hydrophobic groups.
Would electrostatic interactions of the buried peptide group with the surrounding environment destabilize a protein?
Pace argues that this would be more than compensated for by favorable van der Waal's interactions (short range) at the buried
site. This can be illustrate by comparing the DG transfer of an amide from water to the
vapor (11.2 kcal/mol)) compared from water to cyclohexane (7.6 kcal/mol). Transfer to the vapor is more unfavored (due
to the desolvation required when it moves to the gas phase) than to cyclohexane, even though a cavity must be created in the
cyclohexane (a process which would be unfavored entropically). Transfer to octanol is even more favored (1.4 kcal/mol) but
all these values are still positive (disfavored). Similar experiments with transfer of a methylene group (-CH2-)
are negative, given the hydrophobic effect and the close packing van der Waal's interactions possible. These suggest
that van der Waals interactions formed on burying an amide in any solvent are stabilizing. Now consider the packing
density of atoms for various substances:
Packing Densities
| substance |
packing density |
| water |
0.36 |
| cyclohexane |
0.44 |
| closest packed spheres |
0.71 |
| protein interiors |
0.75 |
From this table it should be apparent that van der Waals interactions (short range) will be more stabilizing in the interior
of the protein compared to the same groups in bulk water (or in the denatured state). Carbonyl groups are more polarizable
than methylene groups, which should contribute to van der Waals interactions.
One other addition. It has been noted that Gly peptides are not very soluble in water. The backbone, even with
the polar peptide bonds appears to be solvophobic. If the backbone
of any polymer can't interact well with the solvent - i.e. the solvent is "poor" - then the backbone interacts
with itself, which drives collapse. If the backbone interacts well with a "good" solvent, it won't collapse as readily.
Results from thermophilic organism: What kinds of modifications are made to the sequence
of a protein as the temperature that the organism thrives increases? A recent study by Szilagyi and Zavodszky (Structure,
8, pg 493, 2000) studied 93 structures of 25 proteins, 29 from organisms that live at elevated temperatures (thermophiles)
and 64 at nominal temperatures (mesophiles). Here are their results:
- the number of H-bonds and secondary structure elements do not correlate with temperature,
but the number of salt bridges do.
- in organisms that thrive at very high temperatures (100oC), few internal cavities were found.
- in those that thrive at intermediate high temperatures (45-80oC) the surface had more polar residues.
- generally there was an increase in weaker ion pairs (increased distance between the charged side chains) in the
hot group, but increases in strong and weak ion-ion bonds in the very hot group.
Kashefi and Lovley recently reported the identification of a bacteria obtained from a hydrothermal vent in the northeastern
Pacific ocean. In a laboratory setting, the strain grew in water at a temperature of 121oC under high pressure.
These are the same conditions used in autoclaves to produce sterile samples. Cell doubling took place under these conditions
in 24 hours. The authors suggest that this strain would be useful to determine molecules and their properties necessary
for such high temperature growth.
|

